NCERT Solution for Class 6 Chapter 5 - Measurement of Length and Motion
Have you ever wondered how we get the dimensions of a pencil or how we identify the speed of a vehicle? Class 6 Science Chapter 5-Motion and Measurement of Distances answers all these questions in a very simple and attractive manner. The chapter teaches the students to comprehend the idea of length measurement through measuring instruments that are commonly used in everyday life, like the measurement scale or the measuring tape and also presents standard units like different types of meters, centimetres, and kilometres. It is also used to interpret the right method and unit to use according to the item being measured.
This Story also Contains
- Download Class 6 Science Chapter 5 - Measurement of Length and Motion Question Answers PDF
- NCERT Solutions For Class 6 Science Chapter 5: Exercise Question and Answer
- Class 6 Science Chapter 5 - Measurement of Length and Motion: Additional Questions
- Class 6 Science Chapter 5 - Measurement of Length and Motion - Topics
- Approach to solve the Class 6 Science Chapter 5 - Measurement of Length and Motion
- Benefits of NCERT Solutions for Class 6 Science Curiosity Chapter 5
- NCERT Solutions for Class 6 Science: Chapter-wise

Measurement of length and Motion Class 6 NCERT Solutions are very important to enable the students to grasp the basic ideas of science in a very easy and systematic manner. Measurement of length and Motion Class 6 solutions provide stepwise answers to the questions, and it would be easy for students to understand concepts such as the unit of measurements, types of movements and practical demonstrations. In line with the CBSE course, the NCERT Solutions for Class 6 Science Chapter 5 - Measurement of Length and Motion contain key topics, notes and meaningful examples that would make the learning process more effective and interesting. The NCERT Solutions of Class 6 Science Chapter 5, include solutions of the Exercise Questions NCERT book to direct students about the right methods to answer, Important topics and Definitions: to understand and to keep concepts in mind, Key Topics, units of measurement, history of transport, and various kinds of motion.
Download Class 6 Science Chapter 5 - Measurement of Length and Motion Question Answers PDF
The Measurement of length and motion Class 6 question-answers PDF provides well-structured and step-by-step solutions to all the textbook exercises. These class 6 science chapter 5 Measurement of Length and Motion question answers are designed to help students clarify concepts, improve accuracy, and prepare effectively for school exams. With an easy-to-download PDF format, learners can revise anytime, making their study more organised and exam-oriented.
NCERT Solutions For Class 6 Science Chapter 5: Exercise Question and Answer
Measurement of length and motion Class 6 question-answers provide clear and precise answers to each of the questions referring to the latest textbook, Curiosity, released within NEP 2020. Measurement of Length and Motion class 6 question answers enable the students to learn the main concepts more profoundly, and the exercises of the textbooks can be solved more easily by studying and revising the material before the exams, and deepening their Science knowledge.
Question 1: Some lengths are given in Column I of Table 5.5. Some units are given in Column II. Match the lengths with the units suitable for measuring those lengths.
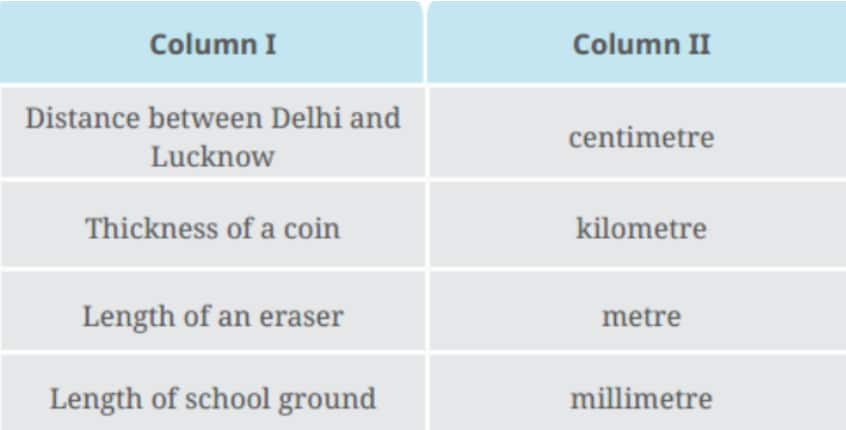
Table 5.5
Answer :
|
Column I |
Column II |
|
Distance between Delhi and Lucknow |
kilometre |
|
Thickness of a coin |
millimetre |
|
Length of an eraser |
centimetre |
|
Length of the school ground |
metre |
Question 2.1: Read the following statements and mark True (T) or False (F) against each.
The motion of a car moving on a straight road is an example of linear motion. [ ]
Answer: True
A car moving straight follows a straight-line path, which is called linear or rectilinear motion.
Question 2.2: Read the following statements and mark True (T) or False (F) against each.
Any object which is changing its position with respect to a reference point over time is said to be in motion. [ ]
Answer: True
When an object changes its position regarding a fixed reference point over time, it is said to be in motion.
Question 2.3: Read the following statements and mark True (T) or False (F) against each.
1 km = 100 cm [ ]
Answer: False
1 km = 1,000 m and 1 m = 100 cm, so 1 km = 1,000 × 100 = 100,000 cm, not 100 cm.
Question 3: Which of the following is not a standard unit of measuring length? (i) millimetre (ii) centimetre (iii) kilometre (iv) handspan
Answer: (iv) handspan
Millimetre, centimetre, and kilometre are all standard metric (SI) units for measuring length. "Handspan" varies from person to person and is not universally accepted as a fixed measurement.
Question 4: Search for the different scales or measuring tapes at your home and school. Find out the smallest value that can be measured using each of these scales. Record your observations in a tabular form.
Answer:
|
Type of Scale, Tape, Device |
Smallest Value of Measurement |
|
15 cm Scale |
1 mm |
|
Flexible Tape |
1 mm, 1 inch |
|
Long Tape Roll |
1 cm, 1 inch |
|
Vernier Calliper (from School Lab) |
0.1 mm |
|
Screw Gauge (from School Lab) |
0.01 mm |
Question 5: Suppose the distance between your school and home is 1.5 km. Express it in metres.
Answer : ∵ 1 km = 1000 metres
∴ 1.5 km = 1.5 × 1000 = 1500 metres
Question 6: Take a tumbler or a bottle. Measure the length of the curved part of the base of the glass or bottle and record it.
Answer: Hint: Use a flexible measuring tape or a piece of string to measure the length of the curved part of the base of the tumbler, then measure the string against a ruler.
Question 7: Measure the height of your friend and express it in (i) metres, (ii) centimetres, and (iii) millimetres.
Answer: Hint: Measure the height using a metre scale and express it in:
- Metres (e.g., 1.4 m)
- Centimetres (e.g., 140 cm)
- Millimetres (e.g., 1400 mm)
Question 8: You are given a coin. Estimate how many coins are required to be placed one after the other lengthwise, without leaving any gap between them, to cover the whole length of the chosen side of a notebook. Verify your estimate by measuring the same side of the notebook and the size of the coin using a 15-cm scale.
Answer: Hint: Measure the diameter of the coin and the length of the notebook. Divide the length of the notebook by the diameter of the coin to estimate the number of coins required. Say the diameter of the coin is 2 cm and the length of the notebook is 18 cm. Then (18/2)=9cm coins can be placed side to side along the length of the notebook. Verify by placing the coins end-to-end and measuring again.
Question 9: Give two examples each for linear, circular, and oscillatory motion.
Answer:
- Linear motion: A car moving on a straight road, an eraser dropping straight down.
- Circular motion: A merry-go-round, the motion of a whirling stone tied to a thread.
- Oscillatory motion: A swinging pendulum, the motion of a metal strip pressed and released.
Question 10: Observe different objects around you. It is easier to express the lengths of some objects in mm, some in cm and some in m. Make a list of three objects in each category and enter them in Table 5.6.
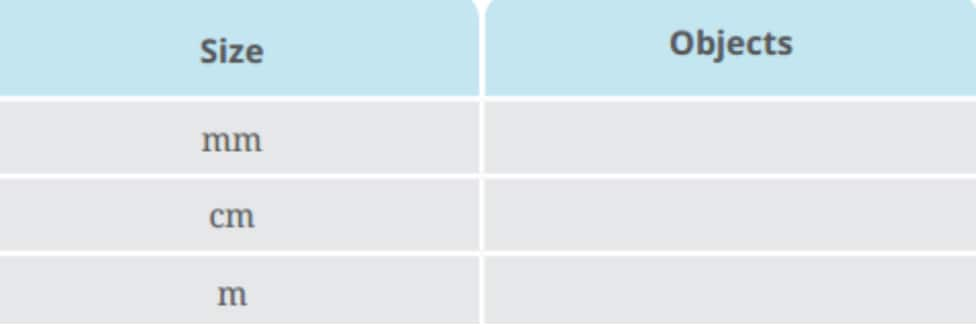
Table 5.6: Sizes of objects around us
Answer: Classify objects by the convenience of measuring in mm, cm, and m:
|
Size Objects |
Size Objects |
|
mm |
Thickness of a coin, thickness of a cardboard and diameter of a small screw |
|
cm |
Length of a pencil, width of a book and height of a water bottle |
|
m |
Height of a room, Width of a playground and height of a lamppost |
Question 11: A rollercoaster track is made in the shape shown in Fig. 5.19. A ball starts from point A and escapes through point F. Identify the types of motion of the ball on the rollercoaster and the corresponding portions of the track.
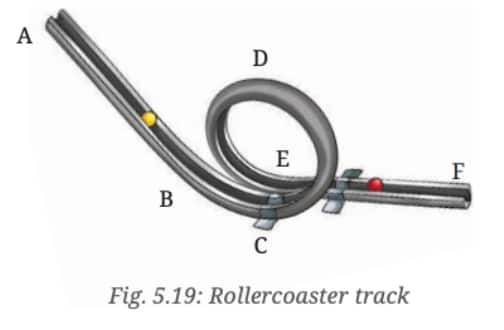
Answer: Portions of the track and corresponding types of motion:
- A to B: Linear motion
- B to C: Circular motion (loop)
- C to D to E: Circular motion
- E to F: Linear motion
Question 12: Tasneem wants to make a metre scale by herself. She considers the following materials for it—plywood, paper, cloth, stretchable rubber and steel. Which of these should she not use and why?
Answer: Tasneem should not use stretchable rubber because it can change length when stretched, leading to inaccurate measurements. Plywood, cloth, paper, and steel are more suitable as they maintain consistent lengths.
Question 13: Think, design and develop a card game on the conversion of units of length to play with your friends.
Answer: Create cards with different lengths and corresponding units (mm, cm, m, km). Each card can have a length in one unit, and players must match it to its equivalent in another unit. For example, a card with “100 cm” would match with “1 m”.
Class 6 Science Chapter 5 - Measurement of Length and Motion: Additional Questions
The additional questions of Class 6 Science Chapter 5 – Measurement of Length and Motion are designed to challenge students beyond the standard exercises. These questions help strengthen problem-solving skills, encourage critical thinking, and provide extra practice on key concepts such as measuring lengths, calculating distances, and understanding motion. Using these questions, students can confidently prepare for exams while applying concepts to practical scenarios.
Q1: Paheli moves on a straight road from point A to point C. She takes 20 minutes to cover a certain distance AB and 30 minutes to cover the rest of the distance BC. She then turns back and takes 30 minutes to cover the distance CB and 20 minutes to cover the rest of the distance to her starting point. She makes 5 rounds on the road the same way. Paheli concludes that her motion is
(a) only rectilinear motion.
(b) only periodic motion.
(c) rectilinear and periodic both.
(d) neither rectilinear nor periodic.
Answer:
Paheli is travelling in a straight path between point A and point C and vice versa, taking the same route several times again and again. Her motion is a straight line (motion along a straight line) since she travels along a straight line (the road) between A and C and back. Since she repeats the same movement (going between A and C and back to A) 5 times, her movement is also periodic because periodic movement repeats after a definite period of time. Accordingly, the movement of Paheli is rectilinear and periodic.
Hence, the correct answer is: (c) both periodic and rectilinear.
Q2: While travelling in a train, it appears that the trees near the track are moving, whereas co-passengers appear to be stationary. Explain the reason.
Answer:
When you are in a train, the trees around the tracks appear to be moving, yet the people inside the train appear to be standing motionless. It occurs due to a phenomenon known as relative motion. Your fellow passengers and you are travelling at the same speed and direction in the same train. And, so, in your opinion, your friends are not moving; they are motionless. But the trees outside are stuck to the ground. The trees appear to be moving backwards as the train is moving past them. In plain words, the appearance of something that is in motion is determined by where one is and at what point of observation. Objects that move with you will appear still, and objects that are passing you will appear to be moving.
Q3: Three students measured the length of a corridor and reported their measurements. The values of their measurements were different. What could be the reason for the difference in their measurements? (Mention any three)
Answer:
Three students who measure the length of a corridor might not record similar results due to the following reasons:
- Measurement Method: They could have applied various methods or tools in order to measure the length. As an example, one might have used a tape measure, the next a meter scale and the third a different length measuring device. Various tools possess varying precisions.
- Starting and Ending Points: It is possible that they did not measure so precisely at the same starting and ending points of the corridor. The slightest change in their starting or ending point can influence the measure.
- Human Factor: The result may vary due to mistakes in the reading of the scale, bending or stretching of the measuring tape or failure to hold the tape straight during measurement.
Class 6 Science Chapter 5 - Measurement of Length and Motion - Topics
The fifth chapter of Class 6 Science, Measurement of Length and Motion, exposes students to the fundamental ideas of distance measurement and movement of objects. It covers common units of measure, measurement techniques and various forms of movement in an easy and practical manner.
5.1 How do we measure?
Measurement is the relationship between an unknown amount and a known reference value. In everyday life, we measure length with the help of different devices such as scales, measuring tape, or rulers. Hand spans or footsteps used in ancient times were not accurate. In science, a standard procedure is desired. This subject brings forth the accuracy of measurement.
5.2 Standard Units
The standard units are units that are permanently defined to be used by everybody in the world. The standard unit of length is the metre (m). Standard units eliminate confusion and give reliable measurements. Smaller distances are represented using sub-units such as centimetre (cm) and millimetre (mm). This part discusses the reason why a global standard is significant.
5.3 Correct Way of Measuring Length
When we measure, we need to employ the right procedures in order to obtain good results. The scale must be lined up along the object, and the eye line must be horizontal so as not to cause parallax error. Damaged or imperfect scales must not be used. This section assists the learners in knowing how to measure.
5.4 Measuring the length of a curved line
As opposed to straight lines, curved lines cannot be measured with an ordinary ruler. A strip of thread or a piece of string is drawn on the curve, and it is measured on a scale. In this section, a non-linear path measurement using simple tools will be described.
5.5 Describing Position
Position can be defined as the place of an object on the basis to a fixed point or with reference. Many times, we define position in terms of directions or coordinates. In science, position must be described accurately when one wants to observe motion or carry out experiments. This subject prepares the ground, which deals with motion and place.
5.6 Moving Things
In this section, the concept of motion is discussed. Things that appear to shift position with time are referred to as moving. Examples would be cars, pets, or even the hands of a clock. It assists the students in learning the role of movement in human daily lives and its importance of knowing motion.
5.7 Types of Motion
Different types of motion include rectilinear (straight line), circular, as well as periodic motion. A car travelling on the road depicts rectilinear motion, and the four-blade fan rotating in a circular motion. Periodic motion takes place after a certain interval and then repeats itself, such as a swinging pendulum. This subject classifies motion in order to develop more knowledge.
Approach to solve the Class 6 Science Chapter 5 - Measurement of Length and Motion
Class 6, chapter 5: Measurement of Length and Motion presents students with the idea of measurement of objects or motion in everyday life. In order to answer questions in an effective manner, the students should adopt a structured method. This approach ensures students grasp both theoretical and practical aspects of measurement and motion, helping them answer questions accurately in exams.
- Start by reading the chapter carefully and learning about the standard units of length, measuring tools, vernier calliper, ruler, measuring tape and concepts of motion, speed, and distance.
- Pay attention to significant concepts such as metre, centimetre, kilometre, millimetre, distance, displacement, speed, and uniform and non-uniform movement.
- Relate the measurement and motion concepts with real-life examples, not only to measure the height of a door, the length of a table, or note the speed of a ball rolling, but also to notice how the ball rolls.
- Solve numerical questions involving addition, subtraction, or conversion of units, as well as calculating speed using the formula: Distance = Speed x Time
- Try to label diagrams wherever necessary, e.g. displacement, scales of measurement, or tracks of moving bodies.
- Go through the NCERT Solutions and answer other questions to strengthen the knowledge and to know how to present the answers.
- Try to carry out easy experiments such as measuring the length of various objects with the usage of different items or timing some moving objects to reinforce practice knowledge.
Benefits of NCERT Solutions for Class 6 Science Curiosity Chapter 5
The NCERT Solutions Class 6 Science Chapter 5 - Measurement and Motion of the Curiosity textbook offers the student an easy way of understanding complex concepts through clear and step-by-step explanations. The Class 6 science chapter 5 Measurement of Length and Motion question answers are based on the current NCERT Class 6 Science syllabus (2025-26) and assist students in enhancing their understanding of important scientific concepts on measurement and motion. Through such NCERT solutions, students can be better prepared for exams and have good analytical and problem-solving skills in Science.
1. Proper and Conceptual Answers
The answers to the solutions are well organised, clear and concept-based based and enable the student to understand complicated scientific concepts in an easy manner.
2. In sync with the New NCERT Book Curiosity
These solutions are based on the most recent Class 6 Science textbook, Curiosity, revised for the 2025-26 academic year by NEP 2020, under the National Curriculum Framework.
3. Builds Strong Foundational Understanding
Chapter 5 prepares the foundations of scientific thinking. The solutions also help students to learn relevant concepts using familiar explanations and examples.
4. Useful for Homework and Exam Preparation
These solutions will help the students to have clear confidence in the class assignments, school tests and training on important questions effectively.
5. Encourages Critical Thinking
The solutions are not rote-learning-based because they induce the use of thinking and reasoning capabilities, as well as the ability to apply knowledge.
6. Simplifies Complex Concepts
Complex ideas are simplified into simpler components using diagrams and step-by-step explanations without making learning tedious or a waste of time.
7. Enhances Self-Study
These solutions are very useful in cases of students who need to revise or prepare without external support, considering it more like a self-help manual.
NCERT Solutions for Class 6 Science: Chapter-wise
The NCERT Solutions to Class 6 Science give answers to all the chapters in the recent NCERT textbook in detail, step-by-step. All these solutions enable students to develop sound fundamentals, be well prepared in exams, and learn concepts easily. It is easy to revise and/or access any topic in a matter of seconds through chapter-wise links.
NCERT Solutions for Class 6: Subject-wise
Also Check NCERT Books and NCERT Syllabus here
Frequently Asked Questions (FAQs)
Chapter Five, Measurement of length and motion, shows the value of measuring things and quantities exactly. It describes standard units of measurement and instruments used to measure length, mass and time.
The answers are provided in a very clear and concise manner which are explained step by step in the same pattern as that of Exam. They assist students in various types of questions and enhance their accuracy and confidence.
They detail unit conversion with examples and give a number of common mistakes. Easy techniques combined with straightforward explanations prevent confusion and appropriately convert
Yes, the solutions provide help to carry out book exercises. They assist in performing observations, measurements, and drawing conclusions from hands on activities.
Begin to measure at some other visible mark (e.g. 1 cm) and deduct this mark at the end reading.
The solutions to NCERT are step-by-step with illustrations and proper explanations of how to measure the length perfectly, how to calculate distance and speed, and how to learn the concept of motion in everyday life, which makes students learn and use the concepts more easily.
Students can connect motion with their day-to-day lives, such as riding a bicycle, running or a toy car that is running on a track and this way they are able to see concepts like speed, distance, and time as practical and meaningful.
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
