NCERT Solutions for Class 8 Science Chapter 7 - Particulate Nature of Matter
Have you ever thought about what everything around us is made of? What makes solids, liquids, and gases different from each other? And why does sugar dissolve in water, but sand does not? The answer to all these questions lies in particulate nature of matter ncert solutions. This chapter helps students explore how all substances are made up of tiny particles. This also introduces the basic structure of matter, differences between atoms and molecules, how these particles behave in solids, liquids, and gases and how matter changes its physical state through various methods.
This Story also Contains
- NCERT Solutions for Class 8 Science Chapter 7: Download PDF
- NCERT Solutions for Class 8 Science Chapter 7 ( Exercise Questions with Answers)
- Approach to Solve the Question of Class 8 Science Chapter 7
- Important Questions and Answers from Class 8 Science Chapter 7: Particulate Nature of Matter
- Topic Covered in Class 8 Chapter 7: Particulate Nature of Matter
- What Students Learn from NCERT Solutions for Class 8 Science Chapter 7
- Why Class 8 Science Chapter 7 Particulate Nature of Matter NCERT Solutions are Important?
- NCERT Chapter-Wise Solutions For Class 8 Science
- NCERT Solutions For Class 8 Subject-Wise
- NCERT Books and NCERT Syllabus
NCERT Solutions provide a valuable resource to students for improving their performance in board exams. Our subject matter experts ensure that students can gain maximum knowledge of the chapter. These NCERT Solutions for Class 8 are designed by our experienced subject experts in a very systematic and comprehensive way to develop a clear understanding of complex problems through a series of solved exercise questions and conceptual explanations.
NCERT Solutions for Class 8 Science Chapter 7: Download PDF
Students can download the NCERT Solution for Class 8 Science Chapter 7 particulate nature of matter pdf from the icon given below. These Solutions of NCERT are designed to help you understand the fundamental concepts and solve textbook questions with ease.
NCERT Solutions for Class 8 Science Chapter 7 ( Exercise Questions with Answers)
Find accurate and detailed chapter end particulate nature of matter class 8 question answer given in the textbook. These solutions make it easier for students to revise key concepts and practise different types of questions effectively.
Keep The Curiosity Alive
Question 1. Choose the correct option. The primary difference between solids and liquids is that the constituent particles are:
(i) Closely packed in solids, while they are stationary in liquids.
(ii) Far apart in solids and have fixed positions in liquids.
(iii) Always moving in solids and have a fixed position in liquids.
(iv) Closely packed in solids and move past each other in liquids.
Answer:
The primary difference between solids and liquids is:
Solid particles are always in a close-packed shape and cannot move freely, while liquids are close together and move past each other, allowing liquids to flow freely.
Hence, the correct answer is option (iv).
Question 2. Which of the following statements are true? Correct the false statements.
(i) Melting ice into water is an example of the transformation of a solid into a liquid.
(ii) The melting process involves a decrease in interparticle attractions during the transformation.
(iii) Solids have a fixed shape and a fixed volume.
(iv) The interparticle interactions in solids are very strong, and the interparticle spaces are very small.
(v) When we heat camphor in one corner of a room, the fragrance reaches all corners of the room.
(vi) On heating, we are adding energy to the camphor, and the energy is released as a smell.
Answer:
(i) True: The process of ice turning into water is an example of a solid changing into a liquid, as ice in its solid state melts to become liquid water.
(ii) True: The attraction between particles decreases during melting. As the solid is heated, its particles absorb energy and vibrate more frequently, which allows them to move more freely and transition into the liquid state.
(iii) True: Due to a closed-packed shape, Solids have a fixed shape and a fixed volume, which is a defining property of solids.
(iv) True: Fixed shape and fixed volume are defining properties of solids. That’s why the interparticle interactions in solids are very strong, and the interparticle spaces are very small.
(v) False:
Correct statement: Camphor undergoes sublimation, which means it directly changes from a solid to a gas. These gas particles then diffuse through the air and carry the smell to all parts of the room. Therefore, the spreading of fragrance is not only because of heating.
(v) False:
Correct statement: The smell doesn’t come from energy being released. Camphor undergoes sublimation, which means it directly changes from a solid to a gas. The vaporized particles of camphor disperse into the air, and these particles produce the fragrance, not the energy itself.
Question 3. Choose the correct answer with justification. If we could remove all the constituent particles from a chair, what would happen?
(i) Nothing will change.
(ii) The chair will weigh less due to lost particles.
(iii) Nothing of the chair will remain
Answer:
A chair is made up of tiny particles such as atoms and molecules. If all of these particles were removed, then there would be no matter left, and the chair would completely disappear. Removing all the constituent particles would leave nothing behind.
Hence, the correct answer is option (iii).
Question 4. Why do gases mix easily, while solids do not?
Answer:
Gases mix easily because they are completely free to move in all directions, which allows gas particles to occupy any available space and makes them mix with other particles easily. While solids have a tightly packed shape and strong intermolecular forces, they can vibrate but can not move freely. That’s why they do not mix easily.
Question 5. When spilled on the table, milk in a glass tumbler flows and spreads out, but the glass tumbler stays in the same shape. Justify this statement.
Answer:
Milk is a liquid; it follows the properties of a liquid. As we know, liquids have a loosely packed shape and are also completely free to move, which allows them to flow. That’s why milk spreads when spilled.
A glass tumbler is a solid; it follows the properties of a solid. As we know, solids have a tightly-packed shape and strong intermolecular forces, and they are not free to move, which does not allow them to flow, resulting in solids having a fixed shape and volume; therefore, they always stay in the same shape.
Question 6. Represent diagrammatically the changes in the arrangement of particles as ice melts and transforms into water vapor.
Answer:
The changes in the arrangement of particles as ice melts and transforms into water vapor:

Question 7. Draw a picture representing particles present in the following:
(i) Aluminium foil
(ii) Glycerin
(iii) Methane gas
Answer:
(i) A picture representing particles present in the Aluminium foil: It is a solid, so it will have a solid-like particle arrangement (regular pattern and close together), which is given below.
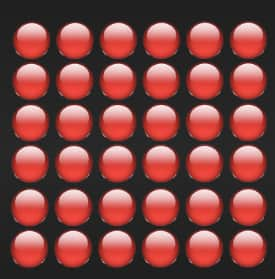
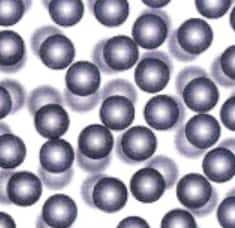
(ii) A picture representing particles present in the glycerin: It is a liquid, so it will have a liquid-like particle arrangement (irregular pattern and close together) , which is given below.
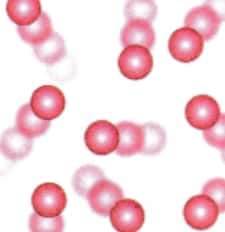
(iii) A picture representing particles present in the methane gas: It is a gas, so it will have a gas-like particle arrangement (irregular pattern and far apart), which is given below.
Question 8. Observe Fig. 7.16(a), which shows the image of a candle that was just extinguished after burning for some time. Identify the different states of wax in the figure and match them with Fig. 7.16(b), showing the arrangement of particles.

Fig. 7.16(a)
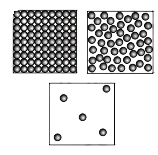
Fig. 7.16(b)
Answer:
Different states of wax:
Solid wax: Unmelted part of the candle
Arrangements of particles in solid wax:

Liquid wax: Near the wick, heat from the flame melts the solid wax into a liquid.
Arrangements of particles in liquid wax:

Vapour wax (Gas): When the candle is burning, due to the high temperature near the flame, some of the liquid wax vaporizes.
Arrangements of particles in vapour wax:
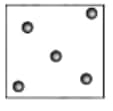
Question 9. Why does the water in the ocean taste salty, even though the salt is not visible? Explain.
Answer:
Ocean water has a salty taste because it contains various dissolved salts, mainly sodium chloride (NaCl). These salts are released from rocks through natural processes. Rainwater dissolves the minerals from rocks, and rivers carry them into the ocean, where the salts have been collecting for millions of years. It is fully dissolved in the water. At the molecular level, it breaks down into tiny sodium and chloride ions, which can not be seen with the naked eye.
Question 10. Grains of rice and rice flour take the shape of the container when placed in different jars. Are they solids or liquids? Explain.
Answer:
Grains of rice and rice flour are solids that have a fixed shape and volume. They do not flow freely like a liquid at the particle level (defining property of solids). However, when large numbers of these solid particles are poured into a container, they settle and spread out, making it look like they are taking the shape of the container, but this is due to their small size and loose arrangement, not because they are liquids.
Approach to Solve the Question of Class 8 Science Chapter 7
Sometimes class 8 science chapter 7 particulate nature of matter question answer seem very difficult, but once we understand the basic rules and strategy, it becomes very easy to solve all the questions related to that particular topic. NCERT Solutions for Class 8 Science can be solved easily by following the steps given below:
1. Before attempting questions, make sure you clearly know the topics, like What are solids, liquids, and gases? The differences between them e.g., defining properties, phase change methods, etc and the types of phase change methods, such as melting, boiling, sublimation, evaporation, etc.
2. Look for words like particle arrangement, structure, shape, solids, liquids, gases, different properties, phase change methods, etc., as they will help you decide whether it is a solid, liquid or gas. You can learn these concepts from the particulate nature of matter ncert solutions.
3. You can use a table to classify the solids, liquids, and gases. This will help you a lot in answering the questions. Also, learn the difference between the phase change methods, such as sublimation, condensation, evaporation, etc
4. Practice as many questions as asked in previous board exams and solve mock tests. Also, solve all the questions given in the NCERT Solution for Class 8 Science Chapter 7 particulate nature of matter to score well in exams.
5. The activities discussed in this chapter are so important and are often asked in exams. Do practice them attentively. Also, focus on true-false and explanation-based questions as they are crucial to understanding.
Important Questions and Answers from Class 8 Science Chapter 7: Particulate Nature of Matter
These class 8 science particulate nature of matter question answer cover the key concepts helping students understand the behaviour of particles and changes in states of matter.
Question 1. Which of the following best explains the particulate nature of matter?
(1) Matter is continuous
(2) Matter is made up of tiny particles
(3) Matter cannot be divided
(4) Matter has no mass
Answer:
Matter consists of very small particles which cannot be seen with the naked eye.
Hence, the correct answer is option (2)
Question 2: Which property of particles of matter is shown by the spreading of perfume in a room?
(1) Particles have mass
(2) Particles are stationary
(3) Particles are in constant motion
(4) Particles have fixed positions
Answer:
Perfume spreads because particles are constantly moving and mix with air particles.
Hence, the correct answer is option (3)
Question 3. What happens to the movement of particles when the temperature of a substance is increased?
(1) Movement decreases
(2) Movement stops
(3) Movement remains same
(4) Movement increases
Answer:
Heating increases the kinetic energy of particles, so they move faster.
Hence, the correct answer is option (4)
Question 4. Which of the following states of matter has the least force of attraction between particles?
(1) Solid
(2) Liquid
(3) Gas
(4) Plasma
Answer:
Gas particles are far apart and have very weak forces of attraction.
Hence, the correct answer is option (3)
Question 5. Which process involves the change of a solid directly into a gas?
(1) Condensation
(2) Sublimation
(3) Evaporation
(4) Freezing
Answer:
Sublimation is the process in which a solid changes directly into a gas without becoming liquid.
Hence, the correct answer is option (2)
Question 6. Why can liquids flow but solids cannot?
(1) Liquids have no mass
(2) Solids have high density
(3) Liquids have weaker intermolecular forces
(4) Solids have no particles
Answer:
Liquids have weaker forces of attraction than solids, allowing particles to slide past each other.
Hence, the correct answer is option (3)
Question 7. Which of the following increases the rate of evaporation?
(1) Decrease in temperature
(2) Increase in humidity
(3) Increase in surface area
(4) Decrease in wind speed
Answer:
A larger surface area allows more particles to escape into the air, increasing evaporation.
Hence, the correct answer is option (3)
Topic Covered in Class 8 Chapter 7: Particulate Nature of Matter
Given below the topics and subtopics covered in class 8 science chapter 7 particulate nature of matter solutions. Students can also refer to the NCERT textbook for a complete understanding of all the topics.
7.1 What Is Matter Composed of?
7.2 What Decides Different States of Matter?
- 7.2.1 Solid state
- 7.2.2 Liquid state
- 7.2.3 Gaseous state
7.3 How Does the Interparticle Spacing Differ in the Three States of Matter?
7.4 How Particles Move in Different States of Matter?
What Students Learn from NCERT Solutions for Class 8 Science Chapter 7
The class 8 science chapter 7 particulate nature of matter solutions provide a clear explanation of the fundamental concept of matter. These solutions simplify complex concepts and help students build a strong foundation for higher studies.
- NCERT Solution for Class 8 Science Chapter 7 particulate nature of matter help students to explains what matter is, and from what it is made of, highlighting that matter is made up of tiny particles that are too small to be seen with the naked eye.
- Here properties of matter, such as diffusion and compressibility are explaines with the help of solved questions.
- These solutions help students to explain the differences between solids, liquids, and gases on the basis of particle arrangement and movement.
- These class 8 science chapter 7 particulate nature of matter question answer are designed in such a way that they help students connect theoretical concepts with real-life examples.
Why Class 8 Science Chapter 7 Particulate Nature of Matter NCERT Solutions are Important?
These particulate nature of matter class 8 question answers provide students with a clear and systematic understanding of how matter is made up of tiny particles. Given below are some points on how these NCERT solutions help students:
1. These solutions help students to understand the behaviour and properties of particles in solids, liquids, and gases.
2. They provide systematic explanations of each question.
3. Using these class 8 science particulate nature of matter question answer help students to cover all important questions which are useful for scoring good marks in exams.
4. Students learn how particle behaviour explains everyday phenomena like melting, boiling, and condensation.
NCERT Chapter-Wise Solutions For Class 8 Science
Besides particulate nature of matter ncert solutions, chapter-wise solutions are given below:
NCERT Solutions For Class 8 Subject-Wise
NCERT Subject-wise solutions for class 8 are given below:
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 8 are given below:
Frequently Asked Questions (FAQs)
The class 8 science chapter 7 particulate nature of matter solutions are step by step answers to the textbook questions. They help students understand how matter is made up of tiny particles, explain its properties, and strengthen conceptual learning for exams.
The key characteristics of particles include that they are very small, have spaces between them, are in constant motion, and have forces of attraction between them.
NCERT Class 8 Science Chapter 7 Particulate Nature of Matter explains that in solids, particles are tightly packed and vibrate in place, making them rigid. In liquids, particles are close but can move past one another, allowing liquids to flow. In gases, particles are far apart and move freely, which explains why gases can occupy larger volumes.
Sublimation: It is a process in which a solid changes directly into a gas without passing through the liquid state. For example, Camphor and naphthalene balls disappear over time because they sublime.
Topics covered in the Class 8 Science Chapter 7 Question Answers?
What is matter composed of?
What decides different states of matter?
How does the interparticle spacing differ in the three states of matter?
How do particles move in different states of matter?
You can identify whether matter is solid or gas by looking at the arrangement and behaviour of its particles. In solids, particles are tightly packed, have a fixed shape, and only vibrate in place. In gases, particles are far apart, move freely in all directions, and have neither a fixed shape nor volume.
Diffusion is the process by which particles spread out from an area of higher concentration to an area of lower concentration. This occurs because particles are always in motion due to their kinetic energy. In gases, particles move rapidly and freely, leading to a quicker diffusion rate compared to liquids and solids, where particles are more restricted in movement.
Temperature is a measure of the average kinetic energy of particles. As the temperature increases, particles acquire more energy, leading to increased movement. This is evident when ice melts to form water and then evaporates to become steam.
The size of particles influences properties like melting and boiling points, density, and solubility. Smaller particles tend to have greater surface area relative to their volume, affecting how they interact with one another. For example, powdered sugar dissolves faster than a sugar cube due to its increased surface area for interactions with water.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
