NEET/JEE Coaching Scholarship
ApplyGet up to 90% Scholarship on Offline NEET/JEE coaching from top Institutes
We use various types of fuels in our day-to-day life, such as the LPG fuel used in the kitchen or petrol, diesel, and CNG in vehicles, wood, which is burnt during winters for warmth, or cow dung cakes that are used for cooking in villages. This NCERT Chapter 4, Class 8, Combustion and Flame, has a detailed discussion on combustion and flame. This chapter includes the different types of combustion, the factors that influence the combustion, the characteristics of flame components involved in the burning process, and the various uses of combustion in our daily lives.

The NCERT Class 8 Science Solutions Chapter 4 Combustion and Flame are designed by our experienced subject matter experts to offer a systematic approach to these important concepts and help students to develop a clear understanding of complex topics through a series of solved examples and conceptual explanations. These solutions also provide a valuable resource to the students to enhance their performance in board exams. Our subject matter experts ensure that through NCERT solutions, students can gain maximum knowledge of the chapter.
Q 1. List conditions under which combustion can take place.
Answer:
The conditions required for the combustion to take place are:
(a) Presence of oxygen
(b) Presence of inflammable substance (fuel)
(c) Maintain Ignition Temperature (the minimum temperature at which a substance catches fire).
Q 2. Fill in the blanks.
(a) Burning of wood and coal causes_________ of air.
(b) A liquid fuel, used in homes, is_________ .
(c) Fuel must be heated to its___________ ___________ before it starts burning.
(d) Fire produced by oil cannot be controlled by__________ .
Answer:
(a) Burning of wood and coal causes pollution of the air.
(b) A liquid fuel used in homes is LPG(Liquefied Petroleum Gas).
(c) Fuel must be heated to its ignition temperature before it starts burning.
(d) The fire produced by oil cannot be controlled by water.
Q 3. Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
Fuels like petroleum and coal release toxic gases like carbon monoxide, carbon dioxide, and sulfur dioxide during combustion. These harmful gases cause pollution and respiratory problems.
Whereas CNG (Compressed Natural Gas) is a clean fuel and produces these harmful gases in very small quantities.
(CNG is methane stored at high pressure!)
Q 4. Compare LPG and wood as fuels.
Answer:
LPG | Wood |
It does not produce smoke. | It produces smoke. |
It is easy to store and can be easily transported using pipelines. | It takes up a lot of space to store. |
It has a low ignition temperature | It has a high ignition temperature |
It does not cause environmental pollution | It leaves unburnt carbon particles, causing air pollution |
The calorific value is higher. | The calorific value is lower. |
Q 5(a). Give reasons.
Water is not used to control fires involving electrical equipment.
Answer:
Water is not used to control fires involving electrical equipment because water, being a good conductor of electricity, may lead the electric current to spread,d causing electric shocks or short-circuits
Q 5(b). Give reasons.
LPG is a better domestic fuel than wood.
Answer:
Wood produces lots of smoke and unburnt carbon particles, which cause respiratory problems. But LPG does not produce these. That's why LPG is a better domestic fuel than wood.
Q 5(c) . Give reasons.
Paper by itself catches fire easily, whereas a piece of paper wrapped around an aluminium pipe does not.
Answer:
Paper has a low ignition temperature and hence catches fire easily. But when it is wrapped around an aluminium pipe, heat is transferred to the metal, and hence the ignition temperature of the paper is not reached. That is why paper by itself catches fire easily, whereas a piece of paper wrapped around an aluminium pipe does not.
Q 6. Make a labelled diagram of a candle flame.
Answer:
Following is the labelled diagram of a candle flame:
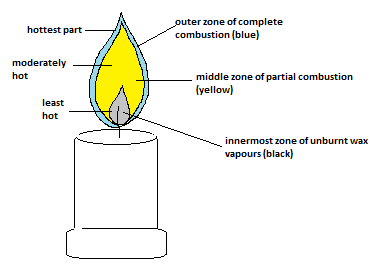
Q 7. Name the unit in which the calorific value of a fuel is expressed.
Answer:
The calorific value of a fuel is expressed in kilojoules per kilogram (kJ/kg).
Q 8. Explain how CO2 is able to control fires.
Answer:
CO2 is heavier than O2 . Therefore, it acts as a blanket over the substance and cuts off the oxygen supply required for combustion. This way CO2 is able to control fires.
Q 9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
It is difficult to burn a heap of green leaves but dry leaves catch fire easily because green leaves have moisture present in them. Hence the heat gets absorbed by water to provide energy to evaporate. But, the dry leaves have no moisture and hence the heat is not lost. That is why it catches fire easily.
Q 10. Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer:
The goldsmith uses the outermost non-luminous (blue) zone for melting gold and silver. This is because it is the hottest part of the flame and hence melts the metals easily.
Answer:
The calorific value of the fuel is the amount of heat produced by the complete combustion of 1 kg of fuel.
Given, Heat produced by 4.5 kg of fuel = 180000 kJ
∴ Heat produced by 1 kg of fuel = 180,000 kJ/4.5 Kg = 40,000 kJ
∴ The calorific value of the fuel is 40,000 kJ/kg.
Answer:
The outermost part of the flame is the hottest region of the flame. Hence, water kept near it will get heated faster, i.e, in a shorter time.
Therefore, the water in Ramesh's beaker will get heated in a shorter time.
In order to prepare this chapter, first read the NCERT Class 8 Science chapter 4, Combustion and Flame. Once you are done with the chapter reading, prepare the NCERT Science Class 8 chapter 4 Combustion and Flame notes and revise them properly. After proper revision, go for the NCERT Science Class 8 chapter 4 Combustion and Flame worksheets. For better preparation, attempt as many NCERT quizzes for Science Class 8 chapter 4 as possible. As far as NCERT preparation is concerned, a great understanding of NCERT solutions for the Science Class 8 chapter 4, Combustion and Flame, can be useful for you.
Apart from combustion and flame class 8 ncert solutions, chapter-wise solutions are given below for science class 8.
| Chapter 1 | Crop Production and Management |
| Chapter 2 | Microorganisms Friend and Foe In Microorganisms |
| Chapter 3 | Coal and Petroleum |
| Chapter 4 | Combustion and Flame |
| Chapter 5 | Conservation of Plants and Animals |
| Chapter 6 | Reproduction in Animals |
| Chapter 7 | Reaching the Age of Adolescence |
| Chapter 8 | Force and Pressure |
| Chapter 9 | Friction |
| Chapter 10 | Sound |
| Chapter 11 | Chemical Effects of Electric Current |
| Chapter 12 | Some Natural Phenomena |
| Chapter 13 | Light |
Combustion is a chemical process in which a substance reacts with oxygen to produce heat and light.
Requirements for Combustion: For combustion to occur, three things are needed: fuel, oxygen, and heat. This is often referred to as the "fire triangle.
Fuel + Oxygen → Carbon Dioxide + Water + Heat + Light
Combustible substances can burn when exposed to heat or a flame, while non-combustible substances do not readily catch fire and burn. Examples of combustible materials include wood and gasoline, while non-combustible materials include metals and glass.
A flame is a visible gaseous part of a fire. It is the region where combustion occurs, and it emits heat and light.
Structure of a Flame
A flame has three zones:

Inner Zone: This is the hottest part of the flame, where complete combustion occurs, and it appears blue.
Middle Zone: In this zone, combustion is incomplete, and it appears yellow.
Outer Zone: The outermost zone contains unburnt fuel, which makes it less hot and less bright.
Types of Flames
Flames can be classified into different types based on the type of fuel and the conditions of combustion. Examples include a blue flame from a Bunsen burner (complete combustion) and a yellow flame from a candle (incomplete combustion).
Fire safety measures include using fire extinguishers, keeping flammable materials away from open flames, and having fire escape plans in place.
Controlling combustion involves regulating the supply of fuel, oxygen, and heat.
Adjusting the air supply can change the colour and temperature of a flame.
Smoke is produced during incomplete combustion and consists of tiny unburnt particles and harmful gases like carbon monoxide.
Fire extinguishers are devices used to control small fires by cutting off the supply of oxygen or cooling the fire. Different types of fire extinguishers are designed for specific types of fires, such as water extinguishers for ordinary fires and CO2 extinguishers for electrical fires.
Topics for class 8 science chapter 4 question answer given below:
Comprehensive Coverage: The class 8 combustion and flame ncert solutions provide comprehensive coverage of the chapter, addressing all important topics and concepts related to combustion and flames.
Step-by-Step Explanations: Each question is explained step by step, making it easier for students to understand the combustion and flame class 8 questions and answers and apply the concepts.
Clarity in Language: The Class 8 science chapter 4 question answers are written in clear and concise language, ensuring that students can grasp the explanations easily.
Conceptual Clarity: These solutions focus on building a strong conceptual understanding of combustion and flames, helping students develop a solid foundation in the subject.
Exam Preparation: The combustion and flame class 8 solutions serve as valuable resources for exam preparation, allowing students to practice and improve their performance in Class 8 Science exams.
Chapter Renumbering: Note that "Combustion and Flame" has been renumbered as Chapter 4 in the CBSE Syllabus for 2025-26.
Also, check the NCERT Books and the NCERT Syllabus here:
Common causes of fire hazards in homes are given below:
Improperly stored flammable liquids, Careless smoking, Unattended cooking, Faulty electrical wiring, Lack of working smoke detectors, Unattended candles, Overloaded electrical outlets.
A candle flame has three main zones, which are given below:
Zone 3 (Non-Luminous Zone): It is the outermost zone. It is almost invisible and the hottest part of the flame. With sufficient oxygen, Complete combustion occurs here.
Zone 2 (Luminous Zone): It is the middle zone, which is typically yellow and bright. Incomplete combustion occurs here.
Zone 1 (Dark Inner Zone): It is the innermost zone closest to the wick. It is the coolest part of the flame, where combustion doesn't occur. It also contains unburnt fuel vapor.
Combustion: It is a chemical process that involves rapid reaction between a substance and an oxidant (usually oxygen) to produce heat and light.
Fire can be extinguished by removing one or more elements of the fire triangle:
Removing the oxygen: Smothering the fire with a foam, blanket, or carbon dioxide.
Removing the heat: Cooling the burning material with water or another coolant material.
Removing the fuel: Physically removing unburnt fuel or letting the fuel burn completely.
Ignition temperature is the minimum temperature required to initiate combustion, without any external spark or flame. It is also called the autoignition temperature.
Application Date:24 March,2025 - 23 April,2025
Admit Card Date:04 April,2025 - 26 April,2025

Get up to 90% Scholarship on Offline NEET/JEE coaching from top Institutes

This ebook serves as a valuable study guide for NEET 2025 exam.

This e-book offers NEET PYQ and serves as an indispensable NEET study material.

As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
Accepted by more than 11,000 universities in over 150 countries worldwide