These solutions help students clearly understand the concepts of solutes, solvents, and solutions through simple explanations and examples. These class 8 science chapter 9 the amazing world of solutes, solvents, and solutions question answer make learning easier and build a strong base. Given below are some points on what students learn from these solutions:
NCERT Solutions for Class 8 Science Chapter 9 - The Amazing World of Solutes, Solvents, and Solutions
Do you know why some things float while others sink, or why salt disappears in water, but sand does not? Let us find the answer through this chapter! These class 8 science chapter 9 the amazing world of solutes, solvents, and solutions ncert solutions talk about the solutions and their types in detail. It will explain how mixtures can be uniform or non-uniform depending on how their components combine. This chapter will help you analyse the real-life processes in a better way.
This Story also Contains
- NCERT Solutions for Class 8 Science Chapter 9: Download PDF
- NCERT Solutions for Class 8 Science Chapter 9 Exercise Questions
- Important Questions from NCERT Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions
- Approach to Solve Chapter 9 Questions Effectively
- NCERT Solutions for Class 8 Science Chapter 9: Topics
- What Students Learn from NCERT Solutions for Class 8 Science Chapter 9
- Importance of Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions
- NCERT Chapter-Wise Solutions for Class 8 Science
- NCERT Books and NCERT Syllabus

The important topics like density, solubility, and the factors affecting them are all discussed in the amazing world of solutes, solvents, and solutions ncert solution. The addition of activities throughout the chapter has made the concepts easier and fun. The NCERT Solutions for Class 8 Science are designed by our experts to help you understand these topics through a series of solved questions. These NCERT solutions will improve your problem-solving skills and will help you score well in exams. We have also included some points so that you can build a good approach for solving the questions. Scroll down to know more.
NCERT Solutions for Class 8 Science Chapter 9: Download PDF
Students can download the NCERT Solutions for Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions PDF from the icon below and access detailed solutions to all the questions present in the textbook. These solutions of NCERT will make it easier for students to understand how different substances mix and help them learn the concepts in a simple way.
NCERT Solutions for Class 8 Science Chapter 9 Exercise Questions
These class 8 science chapter 9 the amazing world of solutes, solvents, and solutions question answer provide clear answers to all the questions in the textbook. These NCERT Solutions for Class 8 are designed to help students understand how solutes, solvents, and solutions work in everyday life.
Keep The Curiosity Alive
Question 1. State whether the statements given below are True [T] or False [F]. Correct the false statement(s).
(i) Oxygen gas is more soluble in hot water than in cold water.
(ii) A mixture of sand and water is a solution.
(iii) The amount of space occupied by any object is called its mass.
(iv) An unsaturated solution has more solute dissolved than a saturated solution.
(v) The mixture of different gases in the atmosphere is also a solution.
Answer:
(i) False - Oxygen gas is less soluble in hot water. It dissolves better in cold water; that is why aquatic life survives better in cooler water.
(ii) False - A mixture of sand and water is not a solution because sand does not dissolve and settles at the bottom. It is a non-uniform mixture.
(iii) False - The amount of space an object occupies is its volume, not mass. Mass is the amount of matter in the object.
(iv) False - An unsaturated solution contains less solute than a saturated solution, so more solute can still dissolve in it.
(v) True - Air is a gaseous solution. Since nitrogen is present in the largest amount in the air, it is considered the solvent, while oxygen, argon, carbon dioxide, and other gases are considered solutes.
Question 2. Fill in the blanks.
(i) The volume of a solid can be measured by the method of displacement, where the solid is __________ in water and the ____________ in water level is measured.
(ii) The maximum amount of _______________ dissolved in _______________ at a particular temperature is called solubility at that temperature.
(iii) Generally, the density ____________ with increase in temperature.
(iv) The solution in which glucose has completely dissolved in water, and no more glucose can dissolve at a given temperature, is called a __________ solution of glucose.
Answer:
(i) The volume of a solid can be measured by the displacement method, where the solid is immersed in water and the rise in water level is measured.
(ii) The maximum amount of solute dissolved in a solvent at a specific temperature is called solubility.
(iii) Generally, the density decreases with an increase in temperature.
(iv) The solution in which glucose has completely dissolved in water, and no more glucose can dissolve at a given temperature, is called a saturated solution of glucose.
Question 3. You pour oil into a glass containing some water. The oil floats on top. What does this tell you?
(i) Oil is denser than water
(ii) Water is denser than oil
(iii) Oil and water have the same density
(iv) Oil dissolves in water.
Answer:
The correct answer is option (ii).
Oil floats on top of water because it is less dense, which means for the same volume, oil has less mass than water. Since denser liquids sink and lighter ones float, water stays below and oil forms a layer on top. This also shows that oil and water do not mix which means they are immiscible liquids.
Question 4. A stone sculpture weighs 225 g and has a volume of 90$\mathrm{cm}^3$. Calculate its density and predict whether it will float or sink in water.
Answer:
Given,
Mass = 225 g, Volume = 90 $\mathrm{cm}^3$
Density = Mass ÷ Volume
= 225 ÷ 90 = 2.5 g/$\mathrm{cm}^3$
Since the water has a density of 1 g/$\mathrm{cm}^3$, therefore, the sculpture is denser than water.
Hence, the stone sculpture will sink in water.
Question 5. Which one of the following is the most appropriate statement, and why are the other statements not appropriate?
(i) A saturated solution can still dissolve more solute at a given temperature.
(ii) An unsaturated solution has dissolved the maximum amount of solute possible at a given temperature.
(iii) No more solute can be dissolved into the saturated solution at that temperature.
(iv) A saturated solution forms only at high temperatures
Answer:
The correct answer is option (iii).
This is because a saturated solution already has the maximum amount of solute it can hold at that temperature. The other options are incorrect because,
(i) A saturated solution cannot dissolve more solute.
(ii) An unsaturated solution is the one that can still dissolve more.
(iv) A saturated solution can form at any temperature, not only at high temperatures
Question 6. You have a bottle with a volume of 2 litres. You pour 500 mL of water into it. How much more water can the bottle hold?
Answer:
The bottle can hold 2 L = 2000 mL.
The amount of water poured = 500 mL
Therefore, the remaining volume is = 2000 – 500 = 1500 mL
So, the bottle can still hold 1500 mL (or 1.5 L) of more water.
Question 7. An object has a mass of 400 g and a volume of 40 $\mathrm{cm}^3$. What is its density?
Answer:
Given,
Mass = 400 g, Volume = 40 $\mathrm{cm}^3$
As we know,
Density = Mass ÷ Volume
= 400 ÷ 40 = 10 g/$\mathrm{cm}^3$
Therefore, the density of this object is 10 g/$\mathrm{cm}^3$, which means the object has 10 grams of mass in every cubic centimeter of space.
Question 8. Analyse Fig. 9.25a and 9.25b. Why does the unpeeled orange float, while the peeled one sinks? Explain.
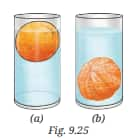
Answer:
The unpeeled orange has a spongy outer peel filled with air pockets that make the whole fruit less dense than water, and so it floats. But when we peel the orange, the air layer is removed, and the orange becomes denser than water, so it sinks. This shows that the presence of air can affect whether an object floats or sinks.
Question 9. Object A has a mass of 200 g and a volume of 40 $\mathrm{cm}^3$. Object B has a mass of 240 g and a volume of 60 cm³. Which object is denser?
Answer:
As we know, Density = Mass ÷ Volume
Therefore, for object A, density (d) = 200 g ÷ 40 $\mathrm{cm}^3$ = 5 g/$\mathrm{cm}^3$
And for object B, density (d`) = 240 g ÷ 60$\mathrm{cm}^3$ = 4 g/$\mathrm{cm}^3$
Since d > d`, therefore, object A is denser than object B.
Question 10. Reema has a piece of modeling clay that weighs 120 g. She first molds it into a compact cube that has a volume of 60 $\mathrm{cm}^3$. Later, she flattens it into a thin sheet. Predict what will happen to its density.
Answer:
Reema has a clay weighing 120 g. She changed the shape of the modeling clay (cube to and thin sheet), but the mass and volume will remain the same. Even though they acquire different shapes, they still have the same amount of matter and will take up the same space.
Since density = mass/volume, and the mass and volume both remained the same, the density will also remain the same. The change in shape does not affect density if the material and volume are unchanged.
Question 11. A block of iron has a mass of 600 g and a density of 7.9 g/$\mathrm{cm}^3$. What is its volume?
Answer:
From the density formula we have,
Volume = Mass ÷ Density
= 600 g ÷ 7.9 g/$\mathrm{cm}^3$
= 75.95 $\mathrm{cm}^3$
So, the volume of the iron block is about 76 $\mathrm{cm}^3$.
This means that 600 g of iron takes up 76 cubic centimeters of space.
Question 12. You are provided with an experimental setup as shown in Fig. 9.26a and 9.26b. On keeping the test tube (Fig. 9.26b) in a beaker containing hot water (~70°C), the water level in the glass tube rises. How does it affect the density?
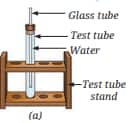

Answer:
When the test tube is placed in hot water, the water inside the glass tube gets heated and expands. This causes the water level to rise. The mass remains the same, but the volume increases.
As we know, density = mass/volume.
That is, d ∝ $\frac{1}{V}$. So, as the volume increases, the density will decrease. This shows that heating reduces density, which is why hot air or water rises.
Important Questions from NCERT Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions
The class 8 science the amazing world of solutes, solvents, and solutions question answer are given below for practice. These practice questions are designed to test students understanding of basic concepts:
Question 1. Which of the following is a solute in a sugar solution?
(1) Water
(2) Sugar
(3) Both sugar and water
(4) None of these
Answer:
In a sugar solution, sugar is the solute because it gets dissolved, while water is the solvent that does the dissolving.
The correct answer is option (2).
Question 2. What is the universal solvent?
(1) Alcohol
(2) Milk
(3) Water
(4) Oil
Answer:
Water is called the universal solvent because it can dissolve more substances than any other liquid, making it essential for life processes.
The correct answer is option (3).
Question 3. When no more solute can be dissolved in a solvent at a given temperature, the solution is called:
(1) Dilute solution
(2) Concentrated solution
(3) Saturated solution
(4) Unsaturated solution
Answer:
A saturated solution is one in which no more solute can dissolve at a fixed temperature. Any extra solute will remain undissolved.
The correct answer is option (3).
Question 4. Which of the following factors increases solubility of solids in liquids?
(1) Decreasing temperature
(2) Stirring
(3) Adding more solute
(4) None of these
Answer:
Stirring helps distribute solute particles evenly throughout the solvent, allowing them to dissolve faster and increasing solubility.
The correct answer is option (2).
Question 5. In a saltwater solution, water acts as the:
(1) Solute
(2) Solvent
(3) Suspension
(4) Sediment
Answer:
In a saltwater solution, water dissolves the salt, so it acts as the solvent.
The correct answer is option (2).
Question 6. A saturated sugar solution is prepared at room temperature. What will happen if more sugar is added after heating the solution?
(1) Sugar will not dissolve
(2) Sugar will dissolve partially
(3) Sugar will dissolve completely
(4) Sugar will decompose
Answer:
Heating increases the solubility of sugar in water, so additional sugar can dissolve, making the solution unsaturated again.
The correct answer is option (3).
Question 7. Why does a cold glass of water turn wet when kept in open air?
(1) Water leaks through the glass
(2) Water evaporates from the glass
(3) Water vapour from air condenses on the glass
(4) Oxygen reacts with the glass
Answer:
The cold surface of the glass causes condensation of water vapour present in air, forming water droplets.
The correct answer is option (3).
Question 8. A student filters a mixture of sand and salt dissolved in water. What will be present in the filtrate?
(1) Sand only
(2) Salt only
(3) Sand and salt
(4) Pure water
Answer:
Sand is insoluble and remains on the filter paper, while salt dissolves in water and passes through as part of the filtrate.
The correct answer is option (2).
Question 9. A student prepares a solution by dissolving 20 g of salt in 100 mL of water at room temperature. On adding more salt, it remains undissolved. What will happen if the solution is stirred continuously and heated?
(1) The undissolved salt will remain unchanged
(2) The salt will decompose
(3) More salt will dissolve
(4) Water will evaporate without dissolving salt
Answer:
Heating increases the solubility of salt in water, allowing more salt to dissolve. Stirring helps distribute particles evenly, speeding up the process.
The correct answer is option (3).
Approach to Solve Chapter 9 Questions Effectively
To solve questions from the amazing world of solutes, solvents, and solutions ncert solutions effectively, you need to work on the following points given below.
1. The understanding of the topics is crucial to attempt the questions. Some important topics are
- The types of solution- saturated or unsaturated.
- The type of mixtures- uniform or non-uniform.
Learn these concepts with the help of examples, as they can be asked in exams directly.
2. Density is defined as the mass present in a unit volume of that substance. Learn to apply the formula
Density = Mass ÷ Volume
The solubility of compounds is often asked in exams. The effect of temperature on solubility is one of the most important topics of this chapter. You can learn these concepts by solving the amazing world of solutes, solvents, and solutions class 8 question answers.
3. You can make use of tables and charts to revise the concepts. This will help you in answering the questions effectively. These short notes will help you revise the concepts anytime.
4. The activities discussed in the chapter are so important and are often asked in exams. Do practice them attentively.
5. Solve all the class 8 science chapter 9 the amazing world of solutes, solvents, and solutions question answers. Practice the numericals on the density formula and focus on the units.
NCERT Solutions for Class 8 Science Chapter 9: Topics
The topics and subtopics covered in the textbook are listed below. Students can use the class 8 science chapter 9 the amazing world of solutes, solvents, and solutions ncert solutions to understand these topics in detail.
9.1 What Are Solute, Solvent, and Solution?
9.2 How Much Solute Can a Fixed Amount of Solvent Dissolve?
9.2.1 How does temperature affect the solubility of a solute?
9.3 Solubility of Gases
9.4 Why Do Objects Float or Sink in Water?
9.5 What Is Density
9.5.1 Determination of density
9.5.2 Effect of temperature on density
9.5.3 Effect of pressure on density
What Students Learn from NCERT Solutions for Class 8 Science Chapter 9
-
Using these solutions, students will learn what solution is, how it is formed, and what is the difference between solutes, solvents, and solutions
-
Study different types of solutions such as solid in liquid, liquid in liquid, and gas in liquid and the concept of saturation and unsaturated solutions.
-
These class 8 science chapter 9 the amazing world of solutes, solvents, and solutions ncert solutions help students to understand the factors affecting solubility and how the nature of solute and solvent influences solubility.
-
Here students will learn methods of separation using techniques like filtration, evaporation, distillation, and crystallisation.
-
Using these the amazing world of solutes, solvents, and solutions class 8 question answer they get to know how solutions are used in daily life.
Importance of Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions
The class 8 science the amazing world of solutes, solvents, and solutions question answer help students to understand how different substances mix together to form solutions and how solutes and solvents play vital roles in everyday life. Given below some points on why this chapter is important:
1. This chapter explains what solutes, solvents, and solutions are, and how they interact with each other.
2. Students can relate science to daily activities using these class 8 science the amazing world of solutes, solvents, and solutions question answer.
3. Here students will learn about concentration, solubility, and chemical reactions.
4. In these class 8 science chapter 9 the amazing world of solutes, solvents, and solutions question answer concepts like saturated and unsaturated solutions are explained well with the help of examples.
5. They provide detailed solutions that help students in writing precise answers
NCERT Chapter-Wise Solutions for Class 8 Science
Students can also explore the NCERT Solutions for Class 8 Science Chapter 9 The Amazing World of Solutes, Solvents, and Solutions along with other chapter solutions to build a thorough understanding of all topics.
NCERT Books and NCERT Syllabus
Follow the links below to know about the NCERT books and the NCERT syllabus for class 8.
Frequently Asked Questions (FAQs)
Class 8 Science Chapter 9 NCERT Solutions provide detailed, step by step answers to all the textbook questions. They help students understand the concepts of solutes, solvents, and solutions clearly and prepare effectively for exams.
It teaches students about how substances dissolve, the roles of solutes and solvents in forming solutions, types of solutions, factors affecting solubility, and different methods to separate components of solutions. This chapter helps build a strong foundation for understanding various chemical processes.
A saturated solution has the maximum amount of solute dissolved; it cannot take more at that temperature. An unsaturated solution can still dissolve more solute.
For most solids, solubility increases with temperature; more can dissolve in hot water. But for gases, solubility decreases with heat, so they dissolve better in cold water.
This is because of the difference in their density. The objects float if they are less dense than water and sink if they are more dense.
For example, oil floats on water because it is lighter (less dense) than water.
A solvent is the substance that dissolves another substance to form a solution, and it is usually present in a larger amount. A solute is the substance that gets dissolved in the solvent and is usually present in a smaller amount.
If an unsaturated solution is dissolved at a given temperature, it means that more solute can still be added and dissolved in it. As you keep adding solute, it will continue to dissolve until the solution reaches its saturation point, where no more solute can dissolve at that temperature.
Air is actually a mixture of gases, and it does not act purely as a solvent or a solute. However, if we consider air as a solution of gases, then nitrogen acts as the solvent, and the other gases like oxygen, carbon dioxide, and noble gases act as the solutes dissolved in it.
A concentrated solution contains a large amount of solute in relation to the amount of solvent. For instance, if you mix a lot of sugar into a cup of water, you create a concentrated sugar solution.
Common household solutions include saltwater, sugar water, lemon juice (which contains citric acid dissolved in water), and vinegar (acetic acid in water). Each of these solutions has distinct properties and uses.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
