NCERT Class 8, Science: Chemical Effects Of An Electric Current
We have learned in the earlier classes that some materials allow passage of electricity through it. Such materials are good conductors of electricity. While some other materials do not allow passage of electricity easily, we call them poor conductors of electricity. For example, copper and aluminium allow passage of electricity while rubber and plastic do not allow passage of electricity. So far in the earlier classes, we have done activities to test the passage of electricity for solids. What is the case of a liquid? Do liquids conduct electricity? These doubts are cleared in this NCERT Class 8 Science Chapter 14. The main discussions in the chapter are listed below.
Do Liquids Conduct Electricity?
Chemical Effects of Electric Current
Electroplating
Do Liquids Conduct Electricity?
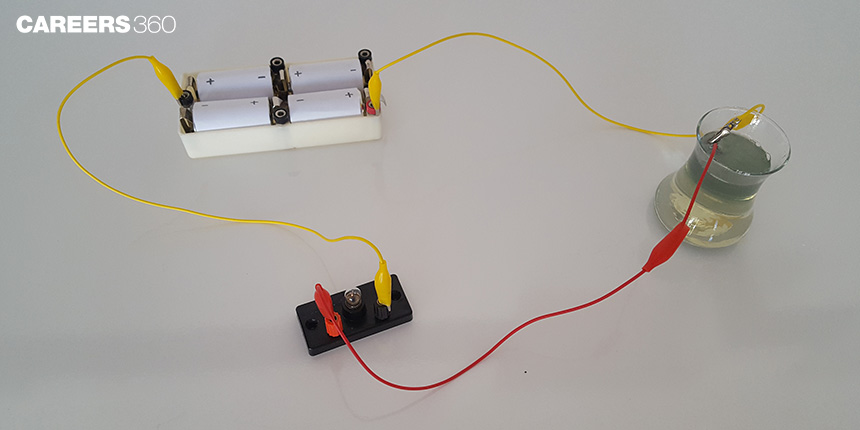
Let us perform an activity to test whether the liquid will conduct electricity.
Activity 1:
Precaution: Do not use electric supply from the mains or a generator or an inverter. Use only electric cells (battery)
Required Materials: A torch bulb or led, connecting wires with insulations, insulation tapes, a battery, lemon juice in a small top of a plastic bottle or rubber top
How to connect: Connections are shown in the figure below.
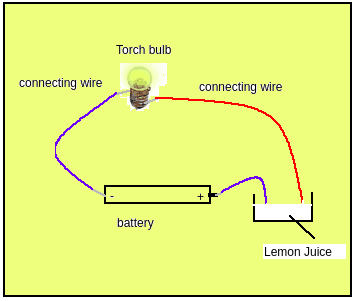 Figure 1
Figure 1
Take a new battery
Connect the negative end of the battery to one end of the bulb. The insulations of the connecting end must be removed, otherwise, the circuit won’t work. Use insulation tape for connection
Connect the positive end of the battery with a wire and keep the other end free
Take another wire(shown as red in figure 1) and connect one end to the bulb as shown in figure 1 with insulation tape. Keep the other end of the wire free. The insulations at connecting ends of wires should be removed.
Now take lemon juice in a small top of a plastic bottle. Dip the free end of blue and red wires in the liquid. (Do not touch the ends of the wire together, if we touch the battery will get drained out fastly). What do you observe? Whether the bulb is glowing?
Conclusion: The torch bulb/led will glow, which indicates that there is a passage of electricity through the lemon juice.
Note: If the torch bulb is not glowing, use led. Since led will glow for a small amount of current also
Tester using the magnetic effect of current:
In the above experiment, we have used a tester with a torch bulb or led. In the earlier classes, we have studied that an electric current produces a magnetic field. If we keep a compass needle near a current-carrying wire the needle deflects even if the current is very small.
Activity 2:
Using the above idea we can construct a tester.
Take an empty matchbox. Remove the upper cover. Place a compass needle inside the matchbox.
Wind the copper wire on it a few times. Now connect one free end of the wire to one terminal of a battery. Leave the other end free.
Take another piece of wire and connect it to the other end of the battery.
Connect the free ends of two wires momentarily. The compass needle should show deflection. Now the tester is ready
Note: Instead of a bulb tester we can use this tester which uses the magnetic effect of current (as shown in Figure 2). This tester can detect a very small amount of current also.
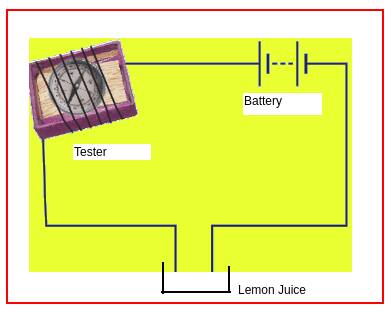 Figure 2
Figure 2
Notes: Instead of lemon juice, we can take tap water, vinegar, milk etc and test whether they will conduct electricity.
Points to remember:
Like solids, all liquids are not a good conductor of electricity. Some are good conductors and some are bad conductors.
We use the word bad conductors instead of insulators because under certain conditions most materials pass electricity. For example, the air is a poor conductor but during lightning, air allows passage of electricity.
Test on distilled water:
Instead of lemon juice mentioned in Activity 1 take distilled water. It is better to use a tester mentioned in Activity 2 or use a tester with led. You can see that the needle does not deflect or if led is used, the led does not glow.
Conclusion: Distilled water does not conduct electricity
Notes:
If we add salt to distilled water then it conducts. In tap, river or well-water many salts are present so it conducts electricity. In distilled water, there is no salt and it does not conduct electricity.
If we add acid or base to distilled water it conducts
Most of the liquids which conduct electricity are solutions of acids, base or salts.
Chemical Effects of Electric Current
When a current passes through a solution there are some changes happening inside the solution. Let us understand this through an activity
Activity 3:
Take out carbon rods with the metal cap from two cells (batteries)
Dip the rod in the water such that the metal cap is outside the water. Add some salt to the water. These rods are called electrodes
Connect copper wires to metal caps and connect it to a cell as shown in Figure 3
Watch the solution near the electrodes
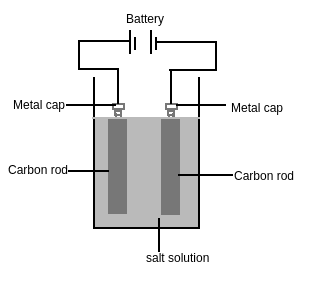 Figure 3
Figure 3
Observations: We can see formations of a bubble near the electrodes. We can say that some chemical changes are occurring in the solution.
Important note: When we pass an electric current through a conducting solution chemical reactions take place inside the solution. As a result of this chemical reaction, bubbles are formed near electrodes, a metal deposit may be seen on electrodes, there may be some change in the colour of the solution. These are some chemical effects of electric current. The effect depends on the type of solution and electrode used.
How to determine the positive terminal of a concealed battery or cell using potato?
Take a potato and cut it into two halves, take one half and connect it to the tester as shown in figure 4. Observe the potato after some time (say 30 minutes). Note down your observation.
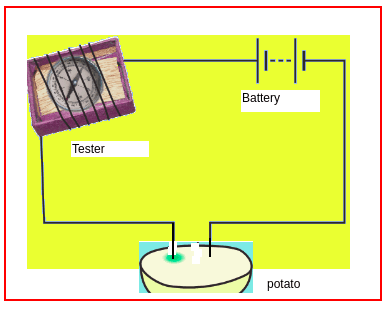 Figure 4
Figure 4
Observation: A greenish-blue spot is observed near the positive terminal of the battery. Thus we can use this experiment to test the positive terminal of a concealed cell.
Electroplating
The process of depositing a layer of any desired metal on another material using electricity is called electroplating. Electroplating is one of the important practical examples of the chemical effect of electric current. To understand how electroplating is done let us see a simple experiment which can be done in the laboratory.
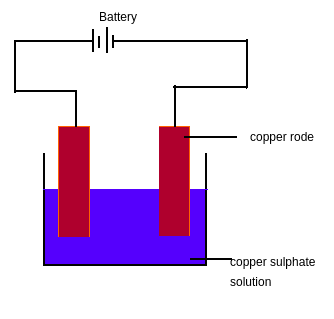 Figure 5
Figure 5
Activity 4:
Take 250 mL distilled water in a beaker.
Add two teaspoons full of copper sulphate in distilled water.
Add a few drops of dilute sulphuric acid to the copper sulphate solution to make it more conducting.
Take two small copper plates (height =10 cm and width= 4cm) and clean them using sandpaper.
Connect the copper plates with a battery
Immerse the copper plates in the copper sulphate solution and allow current for 15 minutes
Remove the electrodes and note down your observations
Observations:
There is a coating of copper on the copper plate connected to the negative terminal of the battery.
The size of the copper plate connected to the positive plate gets reduced.
Reason for the above observations
When we pass a current through copper sulphate solution, copper sulphate dissociates into copper and sulphate. Copper deposits on the negative electrode (that is copperplate connected to the negative terminal of the battery). From the positive electrode, an equal amount of copper gets dissolved in the solution. That is the loss of copper from the solution is restored and the process continues. This means that copper gets transferred from one electrode to the other. Thus copper gets deposited in the negative electrode.
Note: If we do the experiment by replacing the copperplate in the negative terminal of the battery with a carbon rod we can find copper deposits (reddish-brown) on the carbon rod.
Applications of electroplating:
Chromium metal coating on car parts, bicycle handle, kitchen gas burner, wheel rims etc. Chromium does not corrode and has a shiny appearance. Making the whole part with chromium is too costly, so the main part will be made by cheaper metals like iron and a chromium coating will be done on it using electroplating.
Jewellery with electroplating of silver and gold on cheaper metal. Such ornaments will be of low cost and will give the appearance of silver or gold
Tin is less reactive than iron. So to store food tin coating is made on iron cans. So that food will not come in contact with iron and will not get spoilt
To protect the iron from corrosion tin coating is done on iron.
In this chapter, we have learned about the chemical effect of electric current and its applications through activities. The chemical effects of electric current notes given above are useful for conceptual understanding as well as for exams. Also to score well in the exam, practise chemical effects of electric current question answer given in the NCERT book. NCERTsolutions for Class 8 science chapter 14 Will help you in solving questions.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
