Top 9 Named Reactions Of Organic Chemistry
Named reactions in Organic Chemistry generally deal with the synthesis of a specific product using a fixed set of substrate and reagent under predefined temperature and pressure conditions. Mostly the name reactions are named after the person who discovered the particular reaction.
A name reaction follows the basic principles of Organic Chemistry which are discussed in chapter ‘’Organic Chemistry – Some Basic Principles & Techniques’’. Some of the concepts include inductive effect, mesomeric effect, hyperconjugation and more. Mechanism of a name reaction is the most important aspect and each name reaction has a fixed set of steps which must be remembered in order to solve the questions.
In exams such as the Joint Entrance Exam (JEE) Main for Engineering and the National Entrance Eligibility Test (NEET) for medicine, many questions are asked from name reactions. As these questions are straightforward, one can fetch good marks if the important named reactions are understood well.
Detailed here are the top nine named reactions of Organic Chemistry.
1. Pinacol Pinacolone Rearrangement
Pinacol is a compound which has two Hydroxyl groups attached to adjacent Carbon atoms. Pinacol Pinacolone Rearrangement is one of the important reactions of Organic Chemistry which involves the conversion of 1,2 diols into Carbonyl compounds containing Carbon Oxygen Double bond. This reaction takes place via 1,2 rearrangement. The general Pinacol-Pinacolone reaction is depicted below.
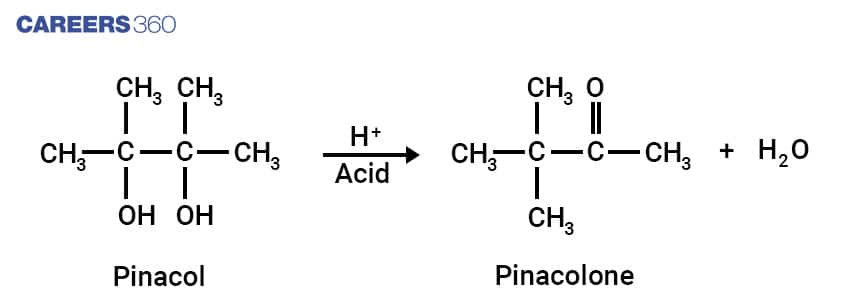
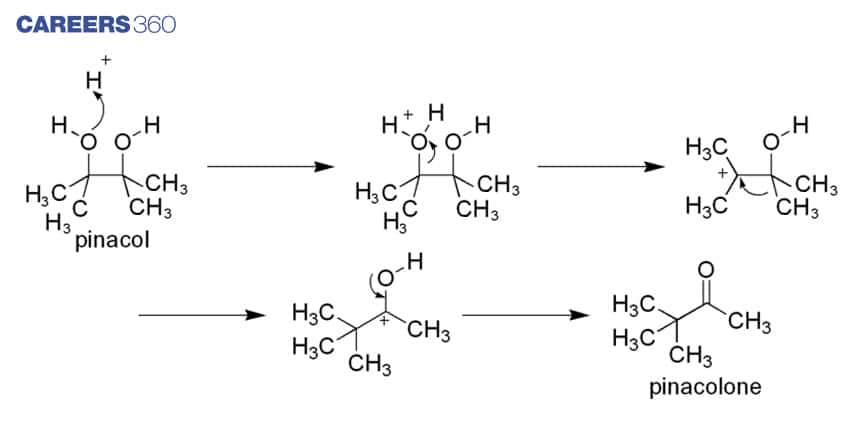
This reaction proceeds via formation of intermediate particles having a positive charge which is caused by the acid used in the reaction. Better migrating group attached to neutral Carbon migrates to fill the vacancy caused by adjacent positively charged carbon atoms. The Oxygen atom is double bonded due to migration which leads to formation of Pinacolone. Mechanism can be depicted below.
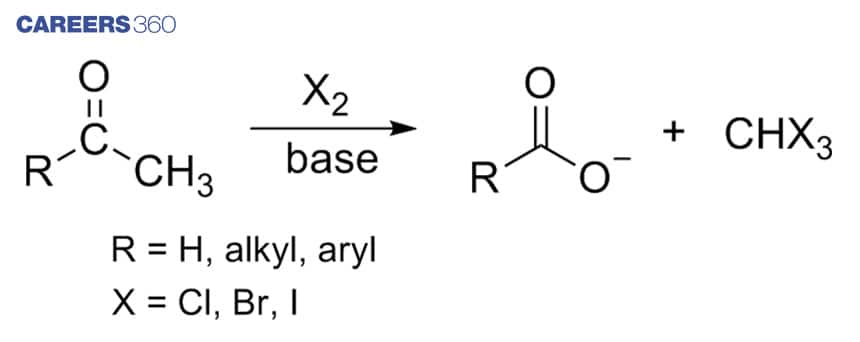
2. Haloform Reaction
Haloform reaction is a type of nucleophilic substitution reaction in which methyl ketone is treated with the bromine or other halogens in aqueous sodium hydroxide solution – Polyhalogenation occurs, followed by the separation of the substituted Methyl group from the ketone. The resulting products of the reaction are the carboxylate and the tribromomethane or bromoform, which is the required haloform. It is to be noted that the reaction is specific to Methyl ketones. Some other functional groups can also respond to the tests like 2-Hydroxy alcohols and acetaldehyde etc,
Below is an example for the haloform reaction.
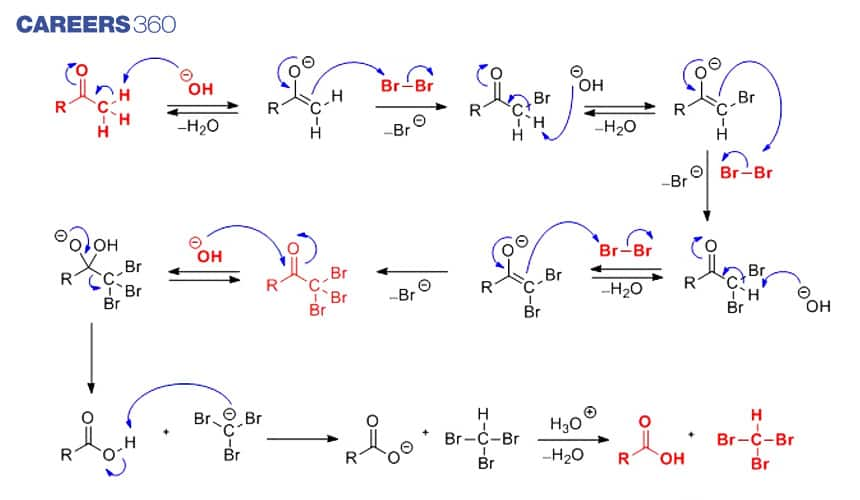
Haloform Reaction Mechanism
Step 1
The hydroxide ion acts as a reagent which takes out the alpha hydrogen producing enolate ion. After this the enolate attack at the halogen which leads to the formation of the halogenated ketone along with the halogens corresponding anion.
Step 2
Further Step 1 is repeated twice to yield a tri-halogenated ketone. The net reaction after 3 times attack of halogen produces tri-halogenated ketone.
Step 3
At tri-halogenated ketone, the hydroxide ion attacks as a nucleophile at electrophilic carbon which is doubly bonded to oxygen. This carbon-oxygen double bond becomes a single bond making the oxygen atom anionic. After this, reformation of the carbon oxygen double bond takes place and the carbon attached to three halogens is displaced,leading to the formation of carboxylic acid. An acid base reaction ensues, the carboxylic acid donates a proton to the tri-halomethyl anion giving the required haloform product.
The overall step by step mechanism is depicted below-
3. Wurtz-Fittig Reaction
Wurtz-Fittig reaction refers to the reaction of aryl halides with alkyl halides and sodium metal in presence of dry ether to form substituted aromatic compounds.This reaction is based on the free radical mechanism. Below is the Wurtz-Fittig reaction which depicts the formation of substituted aromatic compound.
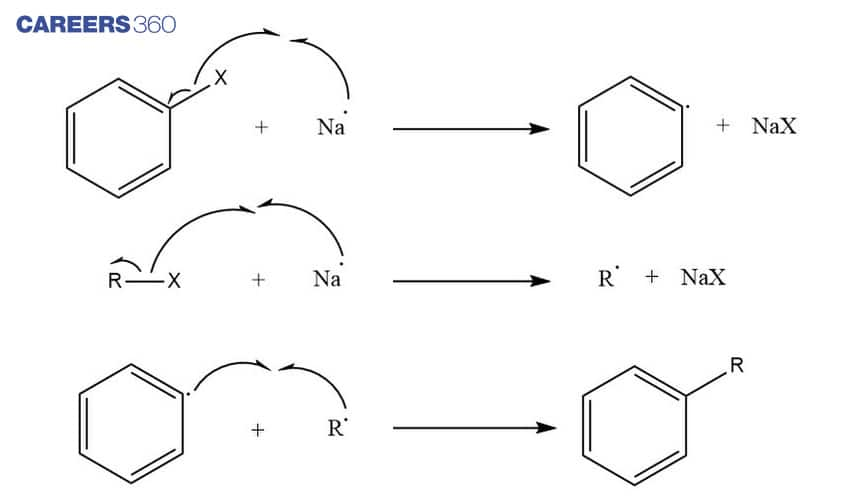
Mechanism
In the Wurtz-Fittig reaction, the Sodium atom acts as a moderator for the formation of alkyl radicals and aryl radicals. These alkyl and aryl radicals combine to form a substituted aromatic compound as shown below.

This reaction can also be called a coupling reaction as it involves the combination of two free radicals.
4. Aldol Condensation
Aldol condensation reaction refers to the reaction of aldehydes or ketones having α-hydrogen with a dilute base to give β-hydroxy aldehydes or ketones. β-hydroxy aldehydes are known as aldols.
In Aldol Condensation reactions, enolate ion is formed by the reaction of a dilute base with aldehyde which further reacts with a carbonyl compound to form β-hydroxy ketone or β-hydroxy aldehyde. Further dehydration takes place in β-hydroxy ketone or β-hydroxy aldehyde in the presence of heat which gives a conjugated enone also called an \alpha-\beta unsaturated carbonyl compound. General reaction of Aldol condensation is depicted below-
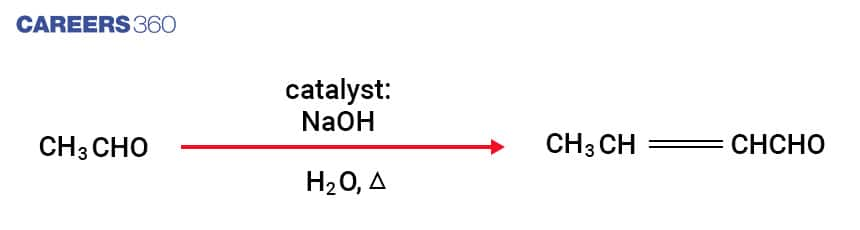
Below is the step by step Mechanism of Aldol Condensation
Step-1:
The hydroxide ion deprotonates the aldehyde forming enolate ion-

Step-2:
Enolate ion formed in step 1 further reacts with unreacted aldehyde forming an alkoxide ion.
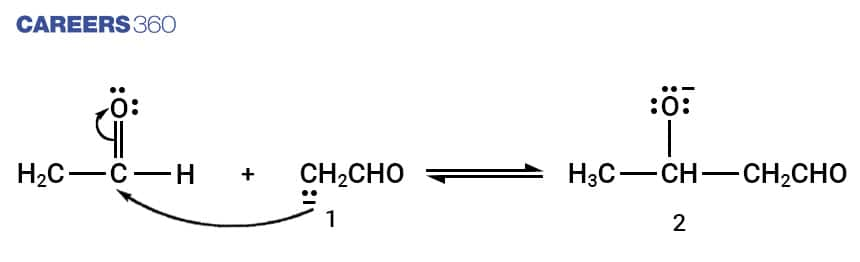
Step-3:
Alkoxide ion formed in step 2 is protonated by water forming β-hydroxy aldehyde also known as aldol.
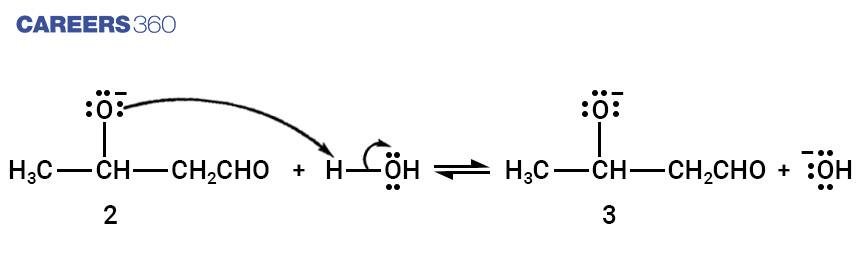
Step-4:
A small amount of β-hydroxy aldehyde formed in step 3 is converted into enolate ion by hydroxide ion.
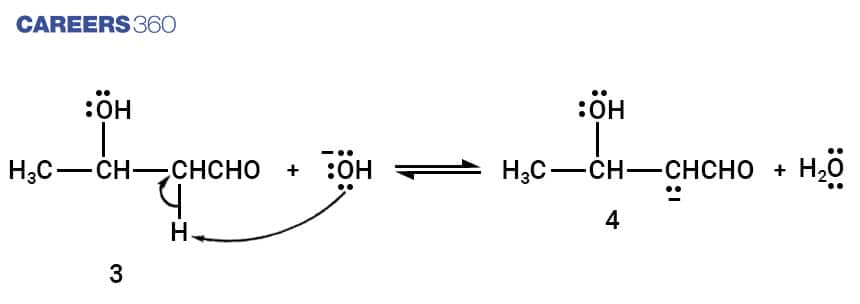
Step-5:
Enolate Ion formed in step 4 loses a hydroxide ion forming a conjugated enone.
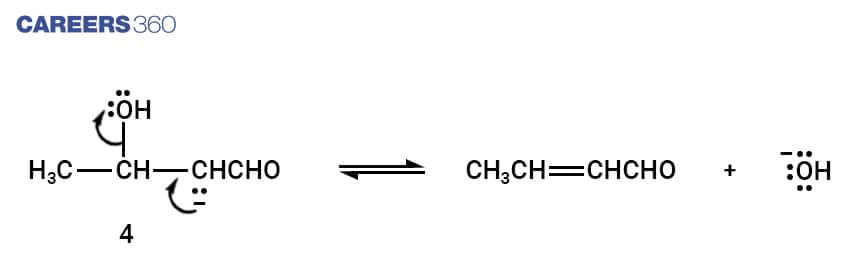
The above reaction steps sums up the aldol condensation reaction.
5. Cannizzaro Reaction
Cannizzaro reaction is the disproportionation reaction of two molecules of non-enolizable aldehyde which yields a carboxylic acid anion and a primary alcohol.
Cannizzaro reaction takes place in concentrated basic medium. The Cannizzaro reaction mechanism details the step by step method of disproportionation reaction of non-enolizable aldehyde which is discussed in detail below.
Step 1
Non-enolizable aldehyde treated with a hydroxide ion which attacks the carbonyl group of the given aldehyde as a nucleophile, giving a dianion.
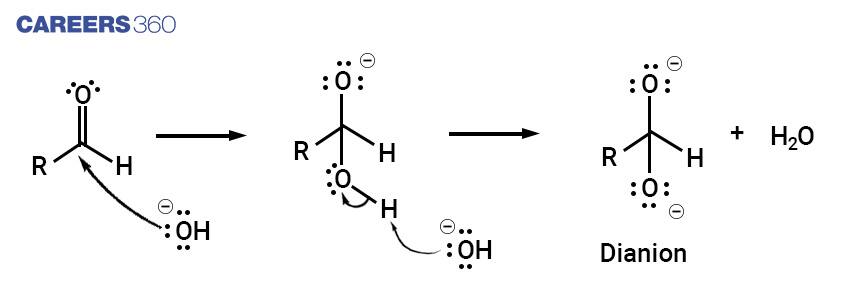
Step 2
The dianion formed in step 1 now acts as a reducing agent by releasing a hydride anion. This hydride anion attacks another aldehyde molecule. The doubly charged anion is converted into a carboxylate anion and the aldehyde is converted into an alkoxide anion.
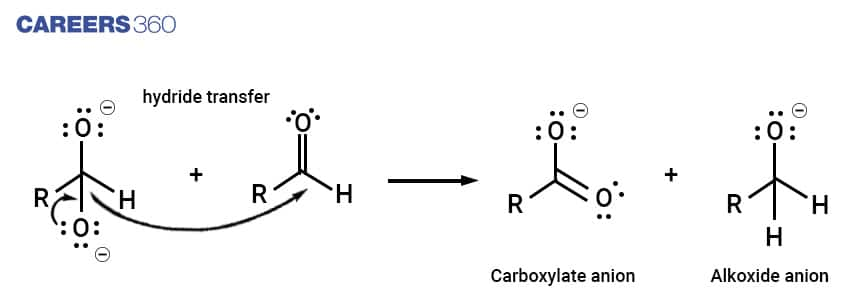
Step 3
Protonation takes place to the alkoxide anion forming an alcohol product. Also the carboxylate ion after protonation gives rise to the Carboxylic acid.
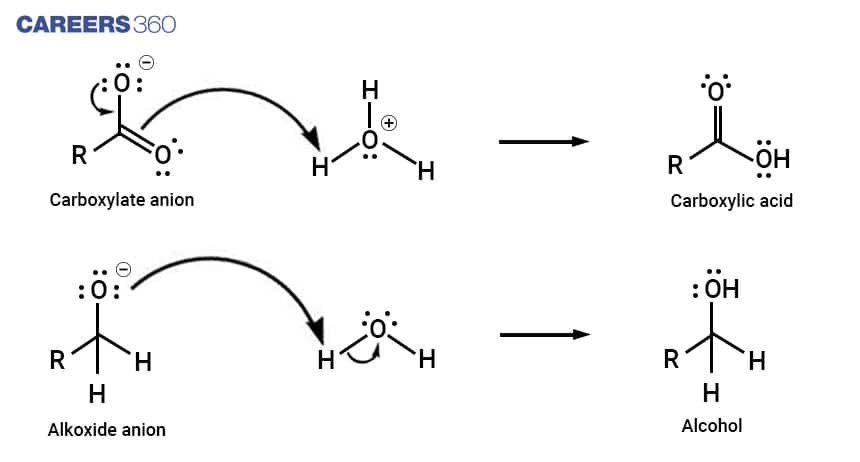
Above mechanism summarises the formation of alcohol and carboxylic acid via Cannizzaro reaction.
6. Reimar-Tiemann reaction
The Reimer Tiemann reaction belongs to the group of nucleophilic substitution reactions. When phenol i.e. C6H5OH is treated with CHCl3 (chloroform) in the presence of NaOH (sodium hydroxide), an aldehyde group (-CHO) is introduced at the ortho position of the benzene ring which leads to the formation of o-hydroxybenzaldehyde. The reaction is popularly known as the Reimer Tiemann reaction.
A common example of the Reimer Tiemann reaction is the conversion of phenol to salicylaldehyde (2-hydroxy benzaldehyde) as shown below.
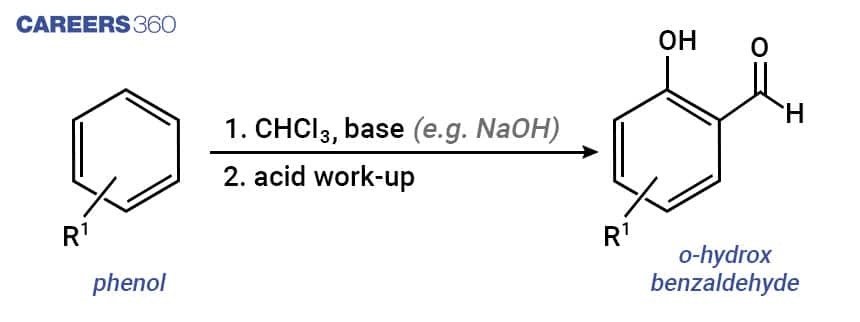
The mechanism of Reimer Tiemann Reaction is detailed below-
First of all the Chloroform is allowed to react with the strongly basic aqueous hydroxide solution leading to formation of chloroform carbanion. The chloroform carbanion readily undergoes alpha elimination, leading to the formation of dichlorocarbene as the reactive intermediate. Dichlorocarbene acts as the electrophile in the Reimer Tiemann Reaction.
The phenol gets deprotonated by the aqueous hydroxide resulting in the formation of a negatively charged phenoxide, this negative charge is delocalised into a benzene ring.
The dichlorocarbene attacks at the negatively charged phenoxide which leads to formation of intermediate dichloromethyl substituted phenol.
The dichloro methyl substituted phenol is subjected to basic hydrolysis which finally forms the ortho-hydroxybenzaldehyde.
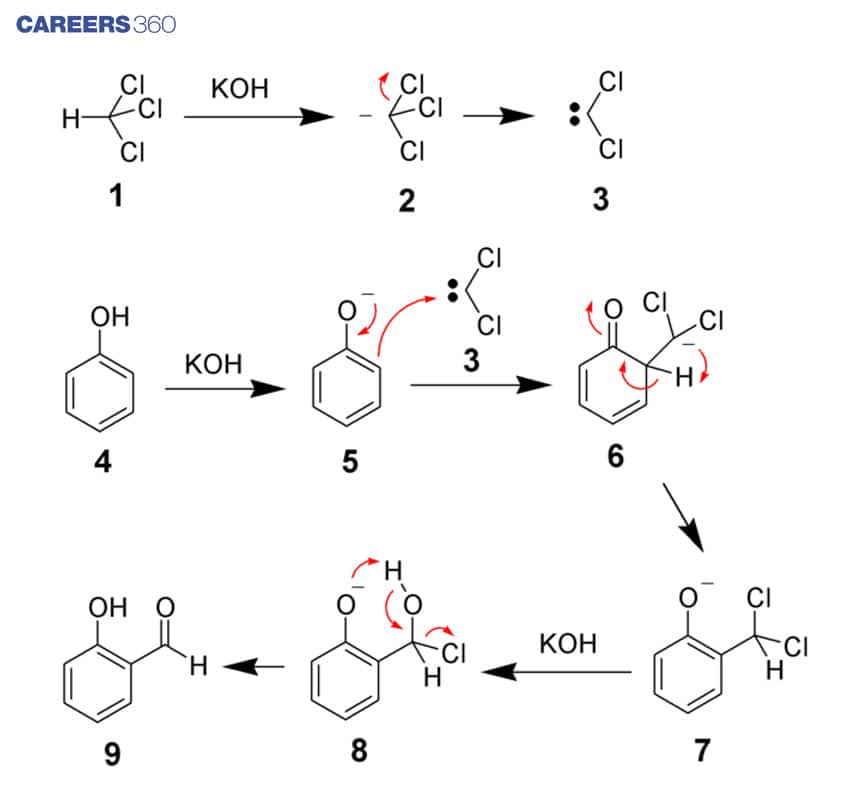
The above reaction mechanism sums up the conversion of Phenol into an ortho-hydroxy benzaldehyde using chloroform and base.
7. Claisen Condensation
Claisen condensation is also named as Claisen Ester condensation is a self condensation reaction of an ester to form Beta- Keto ester. The reaction takes place in the presence of a strong base leading to the formation of a beta-keto ester. Below is the general depiction of the Claisen Condensation reaction.
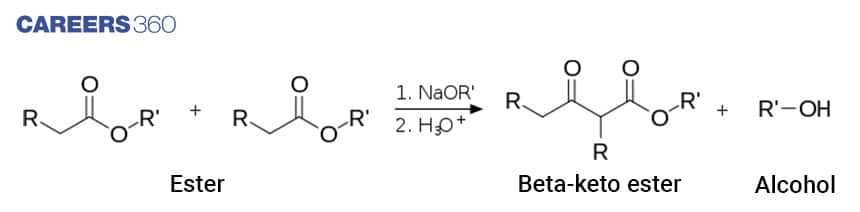
The Claisen condensation reaction requires that a minimum of one reagent must have two alpha protons and can form an enolate anion upon deprotonation.
The reaction also requires the base to avoid participating in nucleophilic substitution reactions or nucleophilic addition with a carbon belonging to the carbonyl functional group.
An ideal base for this reaction is the sodium alkoxide which is the conjugate base of the alcohol to be formed since it is regenerated.
Another requirement is that the alkoxy part of the ester must behave as a good leaving group, as in the case of ethyl and methyl esters.
Mechanism of Claisen Condensation
Step 1
The ester is attacked by the strong base which removes an alpha proton resulting in the formation of an enolate ion.
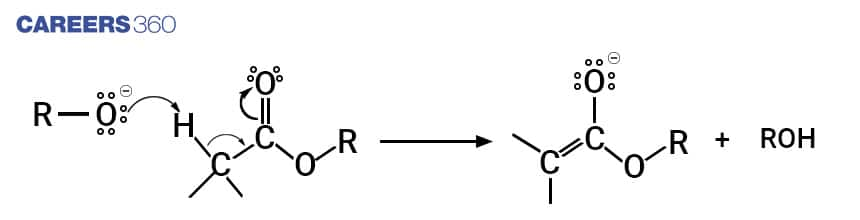
Step 2
In this step the enolate ion already formed, attacks the carbonyl carbon belonging to the second ester as a nucleophilic attack.
Step 3
In this step, the formation of a beta-diketone or a beta-keto ester takes place. This is considered as the final step of Claisen Condensation reaction.

All the above steps depict the Claisen Condensation in which the ester reactants are converted into beta-keto esters or beta-diketones.
8. Sandmeyer Reaction
Sandmeyer reaction is a type of nucleophilic aromatic substitution reaction which is used in the production of aryl halides from aryl diazonium salts. The Sandmeyer reaction can also be used for the transformation of aryl diazonium salts into various other compounds which includes hydroxylation, trifluoromethylation, cyanation, and halogenation.
In the Sandmeyer reaction, the amino group that is attached to an aromatic ring is converted into a diazonium salt which can be transformed into various functional groups.
Sandmeyer Reaction Mechanism
Below mentioned mechanism depicts the formation of Benzenediazonium Ion from Aniline. The formed Benzenediazonium Ion further reacts with HX in the presence of Cu2X2 for halogenation. In this reaction, HX is used where X is a halogen or nucleophile which includes Cl-, I-, CN-, OH- among others.

Below is the single step depiction of Sandmyer reaction which leads to formation of aryl halides directly from the Aniline.
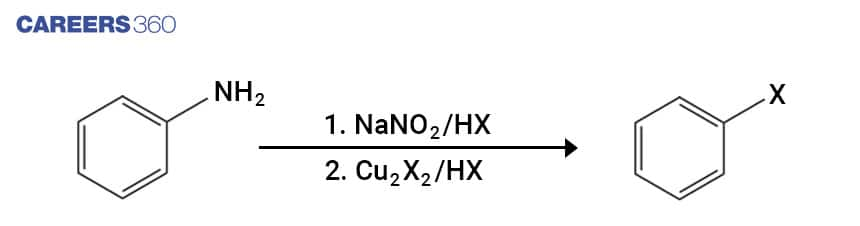
9. Wolff-Kishner Reduction
Wolff Kishner reduction refers to the reaction in which aldehydes and ketones are reduced to alkanes. This conversion takes place in a couple of steps which are discussed below in detail.
Mechanism
Step 1
The aldehyde or ketone taken in the reaction is reacted with hydrazine which leads to the formation of hydrazone.
Step 2
The terminal nitrogen atom in the hydrazone formed in the first step is deprotonated using hydroxide ion and it forms a double bond with the adjacent nitrogen atom. 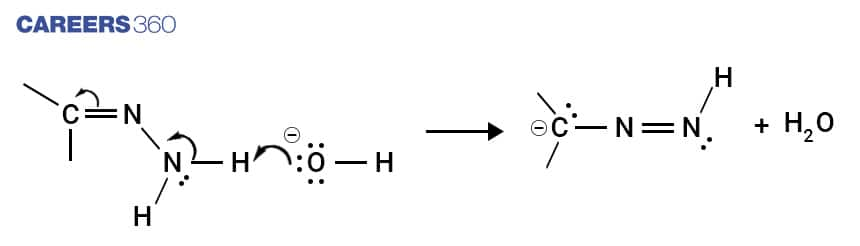
Step 3
In this step the carbon is protonated by the water molecule.
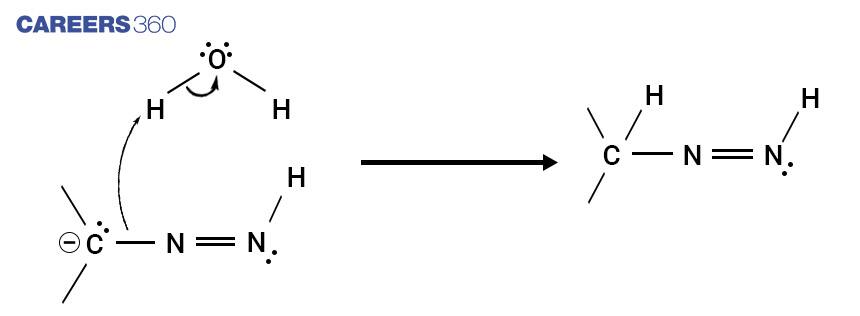
Step 4
The terminal nitrogen is deprotonated again resulting in the formation of a triple bond with its adjacent nitrogen atom which further results in the formation of a carbanion where the two triple bonded nitrogens are released as nitrogen gas.
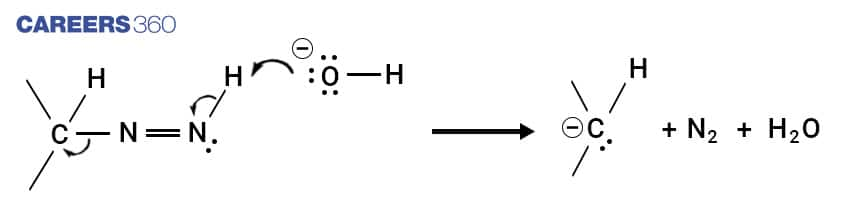
Step 5
The carbon anion formed in the last step is protonated by water, resulting in the formation of the Hydrocarbon product.This is how the aldehyde and ketones are converted into alkanes.
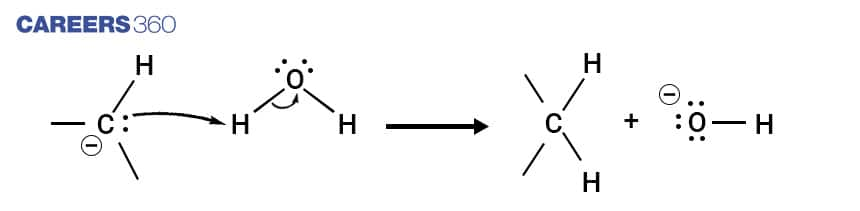
The above reaction mechanism depicts the formation of alkane from aldehydes and ketones via Wolff-Kishner reduction mechanism.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
