Why Do Protons and Electrons Pull on Each Other While They Have Opposite Charges?
Have you ever wondered about the mysterious atoms and their tiny constituents? At the heart of this microscopic realm, protons and electrons play a captivating game of charge and countercharge. You see, protons possess a positive charge, while electrons carry a negative charge.
This Story also Contains
- Coulomb's Law and Attraction Between Charges
- The Structure of an Atom
- Electromagnetic Force and Its Role
- What Stops Protons And Electron Of An Atom From Interacting?
- Real-Life Applications and Implications

Now, here's where the puzzle begins: if opposite charges attract, like magnets pulling together, then why don't these charged particles, protons, and electrons, pull on each other within an atom? Why do atoms remain stable, and not collapse into chaos?
Let's embark on a fascinating journey to uncover the secrets behind why protons and electrons, with their opposite charges, don't collide within atoms! We'll explore the tiny building blocks of matter and discover the fundamental principles that make atoms stable.
Also Check - Take Care Of Your Spine To Live Longer: Class 11 Biology Locomotion And Movement
Coulomb's Law and Attraction Between Charges
Let us begin by explaining Coulomb's Law, which is a magical formula that explains how charged particles behave. It's similar to how gravity draws things together but for electric charges. Assume you have two charged particles, such as protons and electrons. Coulomb's Law states that opposite charges, such as protons (+) and electrons (-), attract one another. It's as if a magnetic force is pulling them together.
Coulomb's Law tells us how strong the force of attraction between protons and electrons is when they are close to each other. It is determined by the amount of charge they have and the distance between them. So, despite the fact that protons and electrons have opposite charges, this equation explains why they are attracted to each other. It's like they're engaged in an invisible tug-of-war!
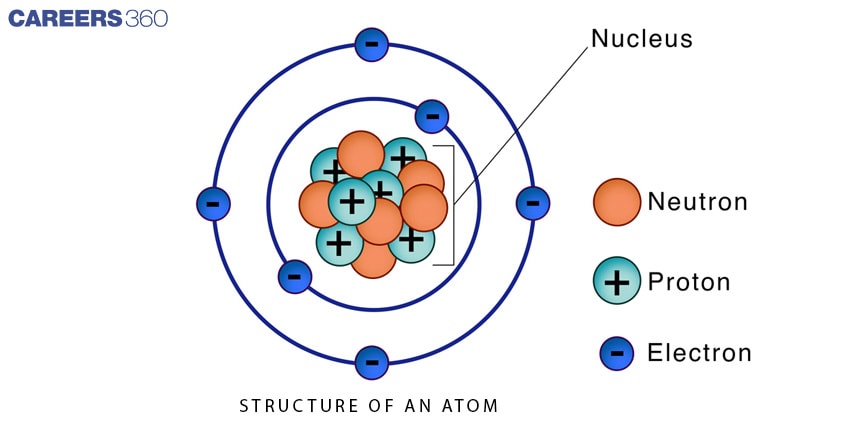
The Structure of an Atom
Atoms are the smallest building units of matter, and their structure is fascinating. They are made up of three basic parts: protons, neutrons, and electrons. The nucleus is made up of protons and neutrons that are closely packed together in the centre of the atom. Electrons occupy a bigger space in various energy levels or shells surrounding the nucleus.
The nucleus is similar to the atom's heart. It is made up of protons, which have a positive charge, and neutrons, which have no charge (they are neutral). Protons and neutrons are heavier than electrons and play an important role in determining an atom's mass and overall stability.
Electrons are much lighter and travel at incredible speeds around the nucleus. They exist at various energy levels, just like planets orbit the Sun. These energy levels are also known as "shells" or "orbitals." The electrons nearest to the nucleus have the least amount of energy, whereas those further away have more.
The arrangement of electrons at various energy levels is critical for the atom's stability. Consider it a dance in which electrons move gracefully in their assigned orbits around the nucleus, and the atom remains in harmony as long as this dance is balanced. Understanding the dance of electrons and their interactions with protons and neutrons is critical for unlocking the mysteries of atomic behaviour and chemical characteristics.
Electromagnetic Force and Its Role
The electromagnetic force is one of nature's fundamental forces, and it is responsible for keeping the atomic world in perfect equilibrium. The electromagnetic force is responsible for interactions between charged particles such as protons and electrons, just as gravity pulls objects towards one other. It acts as an invisible magnet, guiding the behaviour of these tiny particles.
This force is critical in controlling the behaviour of charged particles within an atom. The electromagnetic force, for example, keeps negatively charged electrons "orbiting" around the positively charged nucleus. Without this force, the electrons would either collide with the nucleus or fly away.
The electromagnetic force generates a fascinating interplay of attraction and repulsive forces within an atom. The positively charged protons in the nucleus attract and pull the negatively charged electrons closer together. At the same time, because of their similar charges, negatively charged electrons repel one another. This precise balance of forces is what binds the atom together and keeps its structure and stability intact.
What Stops Protons And Electron Of An Atom From Interacting?
A straightforward experiment can demonstrate that protons and electrons of different atoms interact, while those of the same atom do not. Despite being charged particles with opposite charges, they exhibit selective interactions.
This observation is evident in a simple balloon experiment, where static electricity, an electrical phenomenon, allows charged particles to transfer between objects.
When a balloon is rubbed against hair or sweater, it becomes negatively charged due to gaining electrons. When this negatively charged balloon approaches a wall, the electrons in the wall move away, exposing the protons, which then interact with the negative charges on the balloon.
While electrons from one kind of substance are attracted to protons from another, within the same atom, they do not simply zoom into the nucleus. This is due to the principles of quantum mechanics, where electrons occupy specific energy levels around the nucleus, providing stability to the atom and preventing collapse.
Real-Life Applications and Implications
Understanding the lack of interaction between electrons and protons within an atom is crucial in chemistry, material science, and particle physics. It enables predicting chemical bonding, designing new materials, and studying subatomic behaviour.
This knowledge drives technology and everyday life, powering electronic devices like computers and smartphones. It plays a role in nuclear energy and enhances battery and fuel cell technology. Current research focuses on quantum computing, nanotechnology, and secure communication. Future breakthroughs have the potential to change medicine, electronics, and materials engineering.
Also Check - Understanding The Maths Behind Faraday's Law Of Electromagnetic Induction
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters