Which Is a Smaller Particle - AQuark or An Electron?
In the world of tiny things, there are two very small particles: quarks and electrons. These little things are like the building blocks of everything around us. But here's a puzzling question: which one is tinier, a quark or an electron?
This Story also Contains
- Atoms: The Building Blocks of Matter
- Subatomic Particles: Discovering Electrons and Protons
- Beyond Protons and Electrons: The Neutron Emerges
- The Quark: The Elusive Frontier
- Determining Sizes: A Challenge of Scale

In this article, we're going to explore the world of really small stuff and try to figure out if quarks or electrons are tinier. We'll take a closer look at what these tiny particles are made of, how we know they exist, and use some science magic to understand their sizes. So, let's dive into the world of the very, very small!
Atoms: The Building Blocks of Matter
Our journey begins with atoms, the basic building blocks of matter. Atoms have been thought of since ancient Greece, when philosophers debated the idea of indivisible components. It took generations, however, for the atom to be recognised as a physical fact. Chemist John Dalton's investigations in the nineteenth century revealed the existence of atoms with an average diameter of roughly 50 nano-centimetres - an extremely small size.
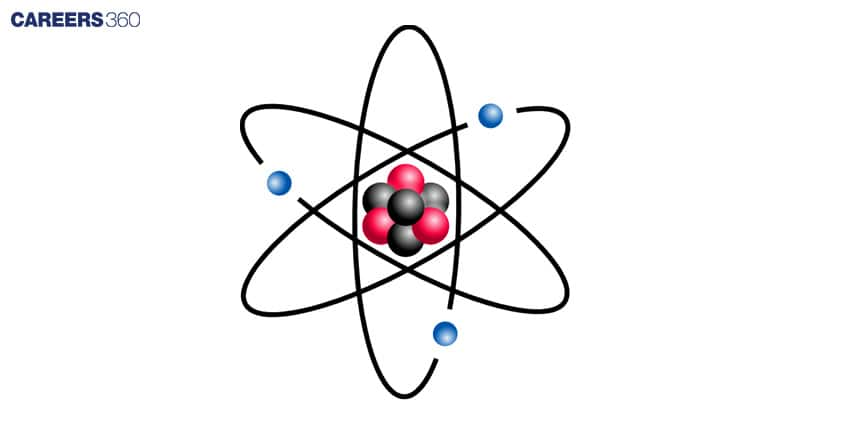
Atoms are the universe's Lego blocks, uniting to produce everything we see around us. They are made up of a nucleus that contains protons and neutrons and is surrounded by electrons. Niels Bohr's model helps us understand how atoms are organised.
Also Read - How Infinite Series Convergence Explains Swinging Motion Of A Pendulum?
Subatomic Particles: Discovering Electrons and Protons
Sir J.J. Thomson's breakthrough discovery of the electron in 1897 showed an even smaller particle, with an average diameter of 0.0000000000001 centimetres - much smaller than an atom. However, this was only the beginning.
Electrons orbit the positively charged nucleus, which contains protons and neutrons, because of their negative charge. This concept, resembling a small solar system, added a new level of complication to the world of subatomic particles.
Beyond Protons and Electrons: The Neutron Emerges
When chemists discovered isotopes - elements with identical protons but different atomic masses - the excitement of discovery was dampened. In 1932, James Chadwick discovered the neutron, an electrically neutral particle with the same size and mass as the proton.
The cornerstone of our understanding of atomic structure is built on neutrons, protons, and electrons. Their presence in an atom's nucleus is critical in defining an element's stability and behaviour.
The Quark: The Elusive Frontier
Quarks were discovered as a result of the search for even more fundamental particles. Physicists witnessed electrons bouncing off protons in particle accelerators revealing three tiny things inside - quarks. These microscopic particles challenged our understanding of protons and neutrons as fundamental building blocks.
Quarks occur in a variety of "flavours" and carry fractional electric charges, defying previously held beliefs about particle size and behaviour. The study of quarks introduces us to quantum chromodynamics, a fascinating field of physics that investigates the strong force that holds quarks together.
Determining Sizes: A Challenge of Scale
The concept of size in the subatomic realm defies our common perception. The concept of shape becomes elusive in the domain of quantum mechanics, and exact measurements are difficult. Quarks, like electrons, are resistant to isolation and survive for only a fraction of a second when separated. Quarks, on the other hand, are thought to be one billionth of a billionth of a centimetre in size, similar to electrons.
When we investigate the concept of size at the subatomic level, we must keep in mind that these measurements are not simple and are based on sophisticated theoretical frameworks and experimental evidence.
In our investigation of the smallest particles, we come upon a world where traditional conceptions of size and shape are challenged. Electrons and quarks, despite their small size, illustrate the complexity of the subatomic realm. While we have achieved tremendous discoveries, the quest to understand the smallest particle is a trip into the unknown, fuelled by curiosity and a never-ending search for knowledge. We continue to peel back the layers of the universe as science advances, seeking answers to issues that challenge our entire understanding of reality.
Also Read- Red Lights: Why Red is the Color of Choice for Signals and Towers Surface?
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
