Quantum Numbers: What Are The Rules for Electron Arrangement?
Think of atoms as tiny puzzles, and quantum numbers as clues that help us solve these puzzles. Quantum numbers are like addresses for electrons inside atoms, telling us where they are and how they behave. Understanding these clues is essential because they reveal why some things stick together, while others don't, in the world of matter.
This Story also Contains
- Quantum Numbers
- Rules for Electron Configuration
- Examples and Applications
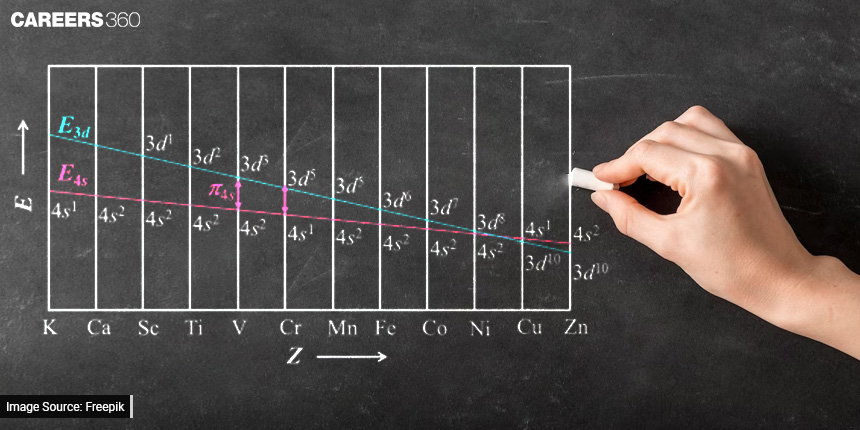
In this article, we'll take a simple journey to discover what quantum numbers are and why they matter. By the end, you'll see how these clues help scientists unlock the secrets of the microscopic world, making it a lot less mysterious.
Quantum Numbers
There are four main types of quantum numbers, each with a unique role in describing where electrons are in atoms. Let's break down what each of them does, so we can understand how they work in the tiny world of atoms.
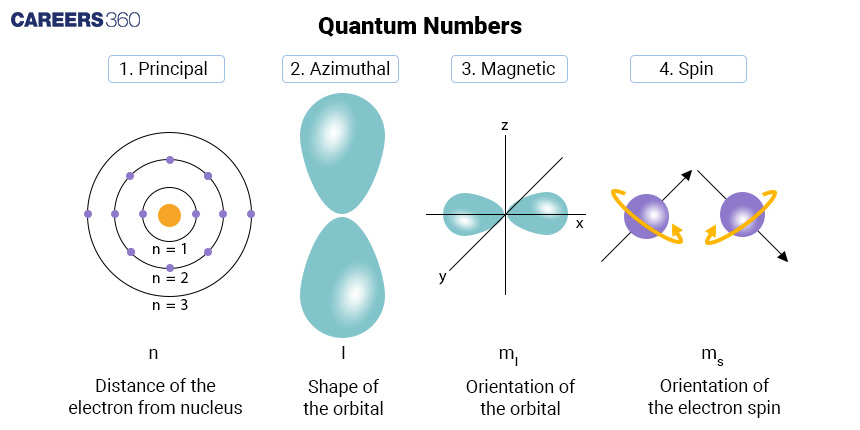
Principal Quantum Number (n): Think of this as the address of an electron within an atom. It tells you which "energy level" or "shell" the electron is in. The higher the number, the farther the electron is from the nucleus. For example, if n = 1, it's in the innermost shell; if n = 2, it's in the next energy level, and so on.
Angular Momentum Quantum Number (l): Imagine each energy level as a structure with various floors, each of which contains rooms of various shapes. The orbital form of an electron's potential home is described by its angular momentum quantum number. It has a range of 0 to (n-1). It resembles a spherical room (s orbital) when l = 0, a more dumbbell-shaped room (p orbital) when l = 1, and so on.
Magnetic Quantum Number (m_l): Now imagine yourself in one of those rooms and asking, "Where is the electron most likely to be?" That is aided by the magnetic quantum number. It provides you with information on the orbital's orientation or direction. Its range of values, from -l to +l, aids in determining the electron's potential location inside the room (orbital).
Spin Quantum Number (m_s): Last but not least, visualise each electron as a small spinning top. The spin orientation of an electron is indicated by the spin quantum number, which can have the values +1/2 or -1/2. It's comparable to understanding if the top is rotating in a clockwise or anticlockwise direction.
Rules for Electron Configuration
Aufbau Principle
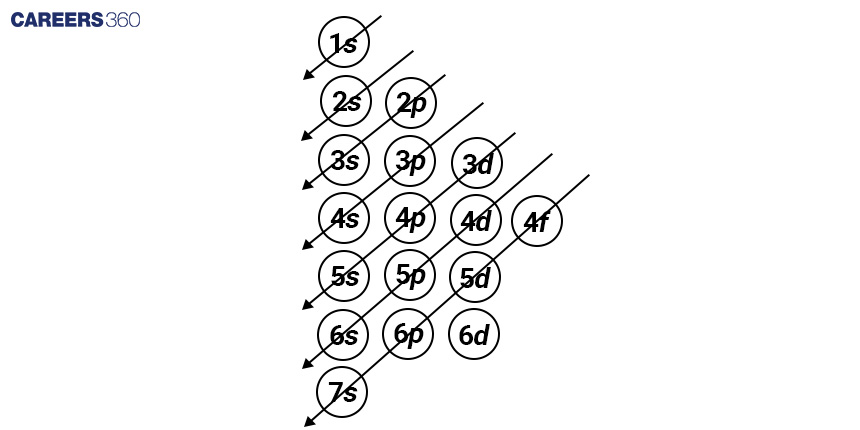
First and foremost, the Aufbau Principle determines the sequence in which electrons occupy atomic orbitals. This theory states that electrons occupy orbitals in decreasing order of energy. Before higher energy orbitals, lower energy orbitals are filled. This rule aids in determining the order in which an atom's electron orbitals—such as the 1s, 2s, 2p, 3s, and so on—are filled with electrons.
Pauli Exclusion Principle
A fundamental law known as the Pauli Exclusion Principle states that no two electrons in the same atom can have the same sets of quantum numbers. To put it another way, it means that each electron needs a certain set of quantum numbers (n, l, ml, and ms). Understanding why there can only be a maximum of two electrons in each orbital, each with an opposite spin (one with "spin-up" and one with "spin-down"), is based on this theory.
Hund's Rule
When dealing with degenerate orbitals—those with the same energy level—with equal n and l values, Hund's Rule is applicable. According to Hund's Rule, electrons will first couple up before entering these degenerate orbitals individually with the same spin (up or down). By reducing electron-electron repulsion inside the atom, this tactic increases its stability. For instance, before any of the three p orbitals (px, py, and pz) begin to pair up, electrons will alone occupy them in the p sublevel (l = 1).
Last but not least, a clear description of how electrons are dispersed among an atom's orbitals is provided by the notation for electron configuration. The primary quantum number (n) and azimuthal quantum number (l) for each orbital are commonly represented by numbers and letters. For instance, the atomic number six (carbon) is represented by the electron configuration 1s22s22p2, which means that there are two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbital. For explaining the electrical structure of atoms, forecasting their chemical behaviour, and comprehending their quantum features, this notation is helpful.
Also check - Scarcity Of Water And Its Impact On Crop Production
Examples and Applications
The electron configuration of an atom has numerous practical applications in chemistry and physics:
>> Chemical Reactivity: An element's chemical behaviour is significantly influenced by its electron configuration. Chemical characteristics of elements with comparable electron configurations frequently overlap. For instance, the electron configurations and subsequent chemical reactivity of elements in the same column (group) in the periodic table are comparable. Predicting how elements will interact in chemical processes requires this information.
>> Magnetism: Electron configuration plays a crucial role in explaining the magnetic properties of materials. Elements with unpaired electrons in their outermost energy levels are often magnetic, while those with all electron spins paired are typically non-magnetic. Understanding the electron configuration helps in designing and using magnetic materials in various applications, including data storage (e.g., hard drives) and medical devices (e.g., MRI machines).
>> Periodic Trends: Atomic size, ionisation energy, and electronegativity are periodic trends in the periodic table that are explained by electron configuration. For instance, whereas elements with a filled valence shell are less likely to form ions and have greater ionisation energies, they are often larger in size and have higher main quantum numbers (n).
>> Chemical Bonding: Understanding the formation of chemical bonds requires knowledge of electron configuration. The octet rule for nonmetals serves as an example of how elements frequently gain, lose, or share electrons in order to reach a stable electron configuration, which is typically one with a full valence shell.
After learning about atoms and quantum numbers, you'll understand more about how the tiniest building blocks of matter work. This knowledge can help you explore exciting things like how different materials react, why some things are magnetic, and why elements in the periodic table behave the way they do. It's like having a superpower for understanding the invisible world of atoms!
Also check - Master The Art Of Problem-Solving With Linear Equations
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
