JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
Polarity Definition: Polarity meaning is the physical qualities of compounds, such as boiling points, melting points, and solubilities, are referred to as polarity. The act among molecules and atoms with different electronegativities is what causes the polarity of bonds.
The definition of polarity is given as: “A state or situation of a molecule with opposite charges, especially when magnetic or electrical poles are present.”
For example take the case of HCl, here the molecule experiences a polarity chemistry due to the opposite charges present on individual atoms:
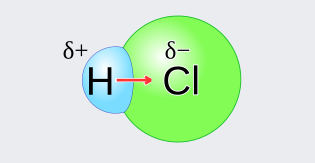
Every molecule has a fixed number of electrons that are organised in a shell at specific energy levels. The valence shell electrons are engaged in chemical bonding with other atoms. To achieve stability, atoms tend to adopt the nobel gas structure. As a result, we can deduce that chemical bonding is responsible for atom and molecule stability and thereby polarity
Also read -
Compounds have a variety of physical properties, such as density, melting and boiling temperatures, solubility, volume, and so on. Polarity is one of the most important features of molecules. The polarity of molecules interferes with their other physical properties. The type of chemical bonding in a molecule, as well as the bound atoms, determine its polarity. We assume that the molecule has polarity if there is a distinct separation of charges.
Ionic and covalent bonds can have polarity. Due to the equal arrangement of chemical bonds, several of the molecules have polar chemical bonds but are nonetheless non-polar in nature. The physical qualities of compounds, such as boiling points, melting points, and solubilities, are referred to as polarity.
The spacing between molecules and atoms with different electronegativities determines the polarity of bonds. The phrase polarity is commonly used in fields such as magnetism, electricity, and electrical device signalling. Consider an electromotive force or electric potential acting between two poles, one of which has a negative polarity and the other has a positive polarity. In Chemistry, polarity refers to the separation of an electric charge that causes molecule to have positive as well as negative end. Consider the illustration below
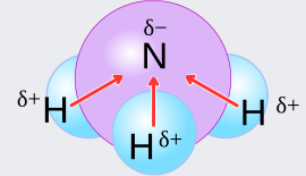
Also read :
The electronegativity of the atoms or molecules determines the bond or molecular polarity. A molecule is usually classified as either a polar, non-polar, or ionic molecule.
Polar Molecules: A polar molecule is generated when one end of the molecule has a higher amount of positive charges than the opposite end, resulting in an electrical pole. When a molecule is stated to have a polar bond, the negative charge centre will be on one side, while the positive charge centre will be on the opposite side.
Non polar molecules: Non-polar molecules are molecules or atoms that have no charges at the end because electrons are evenly distributed and symmetrically cancel each other. A polar molecule cannot be combined with a non polar molecules in a solution. Take, for example, water and oil. Water is a polar molecule, but oil is a non polar molecules in this case.
Related Topics Link, |
Polar or non-polar molecules and atoms exist. A non-polar molecule has its atoms arranged in such a way that the orbital electrons in the outer region cancel out the electronegativity.
Polar molecules are those that have a pyramidal or V-shaped form. Linear molecules, on the other hand, are non-polar.
Because the electronegativity of the oxygen and hydrogen atoms differs, water is classified as a polar molecule. When compared to hydrogen, oxygen is an exceedingly electronegative atom.
Based on the configuration, glucose is another example of a polar molecule.
Non-polar molecules include fats, petrol, oil, and gasoline, which do not dissolve in water and are insoluble in water.
NCERT Chemistry Notes:
Chemical bonds can be of several types based on the participation of atoms and the moving of electrons, such as metallic bonds, covalent bonds, and ionic bonds. Ionic bonds are formed by the electrostatic attraction of two oppositely charged ions. These ions are created when electrons are shifted. An anion is formed when an atom accepts an electron and gains a negative charge, Similarly, when an atom loses an electron, it gains a positive charge and transforms into a cation. The opposite charges of a cation and anion attract one other because electrons entirely transfer to atoms during the establishment of an ionic bond, ions have both negative and positive charges giving rise to a polar molecule.
Proceeding forward, the term polarity is commonly employed in fields such as magnetism, electrical, and electronic device communication. Consider an electromotive force (EMF), also known as an electric potential, that operates between two places. The points, or polarities as they are generally called, have more electrons than another. The pole with the most electrons has negative polarity, whereas the other end has positive polarity.
Covalent bonds are formed when two nonmetals or elements with similar electronegativity come together. For example, the chlorine molecule is produced by bonding atoms exchanging electrons (a electron of each chlorine atom). Two electrons are shared in every covalent link. If an atom requires more than one electron to achieve a stable conformation, it can share the same number of electrons with other atoms.
Also check-
The definition of polarity is given as: “A state or situation of a molecule with opposite charges, especially when magnetic or electrical poles are present.”
In chemical bonding, polarity refers to the distribution of electrical charge across the atoms joined by the bond. Hydrogen chloride, for example, has a slightly positively charged hydrogen atom and a slightly negatively charged chlorine atom.
In chemical bonding, polarity refers to the distribution of electrical charge across the atoms joined by the bond. In hydrogen chloride, for example, the hydrogen atom is slightly positively charged, while the chlorine atom is slightly negatively charged.
Because the electronegativity of the oxygen and hydrogen atoms differs, water is classified as a polar molecule. When compared to hydrogen, oxygen is an exceedingly electronegative atom.
The pole with the most electrons is said to be negative polarity. The pole with the fewest electrons is given positive polarity.. This movement is known as an electric current.
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC

As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

Register FREE for ALLEN Digital Scholarship Admission Test (ADSAT)

Get up to 90% scholarship on NEET, JEE & Foundation courses

As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

Enrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

Start your JEE preparation with ALLEN