JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
![]() chemical name is copper sulfate or copper ii sulfate also known by the name copper sulphate is one of the main inorganic compound. Copper sulfate is generally known as a term which is related with many chemical compounds the main one are cuprous sulfate. The copper sulphate formula is
chemical name is copper sulfate or copper ii sulfate also known by the name copper sulphate is one of the main inorganic compound. Copper sulfate is generally known as a term which is related with many chemical compounds the main one are cuprous sulfate. The copper sulphate formula is ![]() or chemical formula of copper sulphate is
or chemical formula of copper sulphate is![]() . Later for this formula the name mentioned by the scientist is Copper sulfate. Hence from this we can get an idea that systematic name of
. Later for this formula the name mentioned by the scientist is Copper sulfate. Hence from this we can get an idea that systematic name of ![]() is copper sulfate. Other than copper sulphate it is known by the names blue vitriol, roman vitriol, vitriol of copper and bluestone.
is copper sulfate. Other than copper sulphate it is known by the names blue vitriol, roman vitriol, vitriol of copper and bluestone.
One of the most common form of copper sulfate is known as its pentahydrate form. Anhydrous copper sulphate contains no water or moisture. It is dry in texture. The hydrated copper sulphate formula is ![]() known by the name copper sulfate pentahydrate, where penta term corresponds to the number of water i.e. hydra atoms which is 5. The purest form of copper sulfate pentahydrate is blue in color. But if we look about its anhydrous form then this salt is present in white color. Basically it is present in the form of salt which can be shown as below:
known by the name copper sulfate pentahydrate, where penta term corresponds to the number of water i.e. hydra atoms which is 5. The purest form of copper sulfate pentahydrate is blue in color. But if we look about its anhydrous form then this salt is present in white color. Basically it is present in the form of salt which can be shown as below:

Copper sulphate solution is prepared by dissolving copper sulphate in water as it is highly soluble in water.
Also read -
The molecule of copper sulfate contains an ionic bond between cation and anion where cation are positively charged ions and in this case copper is act as cation with +2 charge shown as ![]() and anion is negatively charged ion which is represented by sulfate ion also carries -2 charge represented as
and anion is negatively charged ion which is represented by sulfate ion also carries -2 charge represented as ![]() . Total charge of any compound should be balanced. The structure of copper sulfate can be shown as:
. Total charge of any compound should be balanced. The structure of copper sulfate can be shown as:
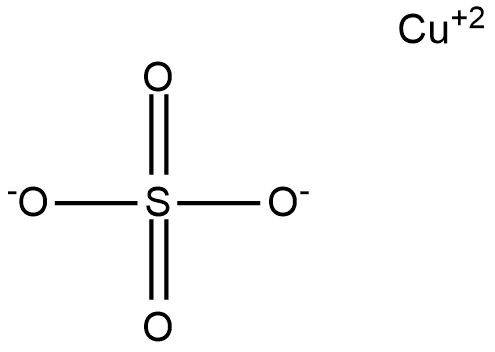
Here copper shows +2 charge while on sulfate two oxygen atom carries -1 charge overall charge is zero. In the similar manner if we talk about copper sulfate pentahydrate represented by the formula ![]() contains five water molecule along with this formula and water molecule are neutral in nature so no effect will be produced by them and the
contains five water molecule along with this formula and water molecule are neutral in nature so no effect will be produced by them and the ![]() structure can be shown as below:
structure can be shown as below:
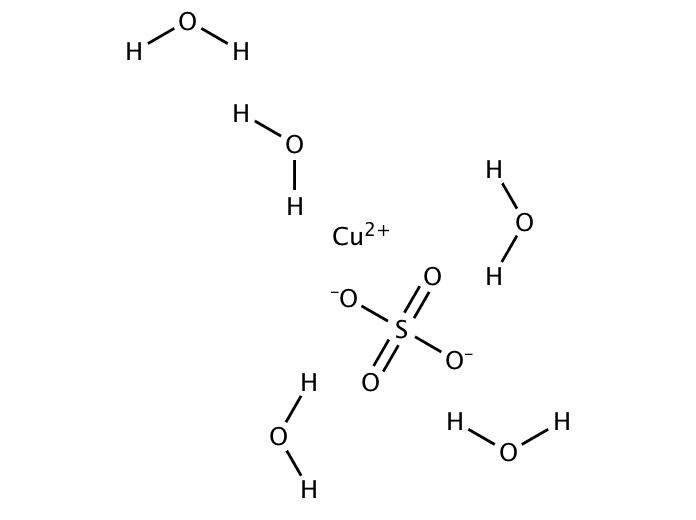
The only difference is involvement of five water molecules.
Preparation of copper sulfate is one of the easiest method it can be prepared by heating metallic copper and reacts it with concentrated sulphuric acid which gives copper sulfate as a product and also liberate hydrogen gas along with copper sufate. Other than metallic copper, copper sulfate can also be prepared by reaction of oxides of copper with dilute sulphuric acid and produce copper sulfate which is blue in color. Oxidation state shown by copper in copper sulfate is +2. The general formula for copper sulfate is ![]() , here x lies in between 0-5. Pentahydrate in which x is 5 is one of the most common form. This pentahydrate form of copper sulfate dissolves in water which gives us aqua complex of formula
, here x lies in between 0-5. Pentahydrate in which x is 5 is one of the most common form. This pentahydrate form of copper sulfate dissolves in water which gives us aqua complex of formula ![]() which shows octahedral geometry and this reaction is highly exothermic in nature while exothermic process is that in which heat is evolved.
which shows octahedral geometry and this reaction is highly exothermic in nature while exothermic process is that in which heat is evolved.
Related Topics link, |
Properties of copper sulfate can be explained on the basis of physical and chemical properties. On this basis properties can be explained as follows:
Physical properties of copper sulfate: Physical properties mentioned the appearance of copper sulfate its shape, boiling point, melting point, solubility etc. These can be explained as follows:
1. Molar mass which is known as the smallest mass unit of any compound corresponds to one twelfth of the mass of carbon. Copper sulfate molar mass is known to us is 159.609 grams per mole and molar mass of copper sulfate penhydrate is 249.685 grams per mole.
2. Copper sulfate is basically present in the form of powdery substance out of which anhydrous form i.e. the form which is free from water molecules is of silvery white color and copper sulfate pentahydrate is present as blue colored powder.
3. The one common property in both anhydrous and hydrated copper sulfate is that they tend to decompose on heating and both will not have exact boiling points.
4. Anhydrous copper sulfate has structure in the shape of orthorhombic while hydrous copper sulfate contains structure of triclinic crystal.
Other than physical properties there are many chemical properties like its different types of reaction with different substances which can be shown as follows:
NCERT Chemistry Notes:
Chemical properties of copper sulfate: The main chemical properties shown by copper sulfate are:
1. Cooper ions present in copper sulfate generally reacts with chloride ions of hydrochloric acid and gives tetrachlorocuprate as a product. This generally involves in two steps in first step hydrochloric acid dissociates into hydrogen and chloride ions which can be shown as:
![]()
After that these chloride ions reacts copper ions gives the product called tetrachlorocuprate here tetra chloro represents the 4 chlorine atom and the reaction can be shown as:
![]()
2. Heating of copper sulfate: When we heat copper sulfate at ![]() then it undergoes through the process called decomposition which yield to give products known by the name cupric oxide shown by the formula
then it undergoes through the process called decomposition which yield to give products known by the name cupric oxide shown by the formula ![]() and sulfur trioxide shown by
and sulfur trioxide shown by ![]() .
.
3. Solubility: Copper sulfate is said to be highly soluble in water. The solubility values of copper sulfates at ![]() and
and ![]() will be 1.055 molal and 1.502 molal respectively.
will be 1.055 molal and 1.502 molal respectively.
The main example of copper sulfate is displacement reaction in which that metal which is highly reactive in nature displace the other metal ion which is of low reactivity it can be shown as:
![]()
This reaction suggests that copper is less reactive than iron therefore it is able to replace copper ion.
Also read :
Copper sulfate shows many type of industrial as well as chemical applications which can be described as follows:
1. Pentahydrate copper sulfate is used as fungicide due to its tendency to kill several types of fungi.
2. The one of main application of copper sulfate is it is used as Benedict solution and Fehling solution in reducing sugars.
3. Copper sulfate also have some medical uses like it used to test blood samples for anemia diseases.
4. Copper sulfate generally mixed with potassium permanganate which further form oxidant.
5. In the process of vegetable dying it is used as a dye fixative.
6. Aqueous solution of copper sulfate can be used as a resistive element for liquid resistors.
7. It can also use for decorative purposes like it add color to cement and other ceramic items and too metal also.
8. It is also added in glues used in binding of books for the purpose of killing insects in printed papers.
Also check-
In case of hydrated copper sulfate the water molecules are surrounding towards the central metal atom copper which further acts as a ligand and go through d-d transitions which emit blue color in visible region and appears blue to us while in anhydrous copper sulfate no transitions can be seen.
Copper sulfate.
Copper sulfate.
It is present as blue color.
Ionic bond.
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC

As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

Register FREE for ALLEN Digital Scholarship Admission Test (ADSAT)

Get up to 90% scholarship on NEET, JEE & Foundation courses

As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

Enrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

Start your JEE preparation with ALLEN