Understanding Density Stratification: Why Does Oil Float On Water?
Have you ever wondered why oil floats on water? It turns out there's a fascinating reason behind it. Think about a rubber duck in a bathtub – it stays on the surface, right? Well, oil does something similar in water, and it's because of something called density.
This Story also Contains
- Density and Buoyancy
- Oil and Water Density Comparison
- Density Stratification
- Archimedes' Principle in Action

Now, density might sound like a big word, but it's just to understand how heavy or light something is for its size. Imagine having a big ball of play dough – if you squeeze it into a small, tight ball, it becomes heavier for its size. That's density!
In this article, we'll explore why oil and water don't mix, quite literally. We'll talk about density, which is all about how crowded things are, and buoyancy, which is like the magic force that decides if things float or sink. So, let's dive in and discover the secrets behind why oil likes to stay on the surface of water!
Also Read- Exploring Energy Storage: Batteries, Capacitors and Beyond For A Greener World
Density and Buoyancy
The density of an object or substance is a measure of how tightly packed the particles are. Consider a crowded bus: if there are many people in a small space, it is crowded; if there are fewer people in the same space, it is less crowded. The same is true for density.
We use a simple formula to calculate density: density equals mass divided by volume. The mass of an object is the amount of "stuff" it contains, and the volume is the amount of space it occupies. So density tells us how much stuff fits into a given amount of space.
Let's talk about buoyancy now. Consider yourself in a swimming pool, attempting to push a beach ball underwater. What happens? The ball keeps reappearing. This is due to buoyancy, which is a force that pushes things up in liquids.
This principle was discovered by Archimedes, an ancient scientist. When an object is immersed in a fluid (such as water), it experiences an upward force known as buoyant force. This force is equal to the weight of the fluid pushed out of the way by the object. So, if the buoyant force is greater than the weight of the object, it floats!
Let us now connect the dots. Consider a super light and fluffy sponge. It has a low density because it is light and takes up a lot of space. When you submerge it in water, the buoyant force overcomes its weight, and it floats!
Consider a large rock, on the other hand. It's dense because there's a lot packed into a small space. When you submerge the rock in water, the weight overcomes the buoyant force and it sinks.
As a result, whether something floats or sinks is determined by the balance of its weight (which is related to density) and the buoyant force pushing up from the water. This equilibrium is what causes oil to float on water; because oil is less dense than water, the buoyant force keeps it at the surface.
Oil and Water Density Comparison
Let's talk about water now that we've figured out density. The density of water is approximately 1 gram per cubic centimeter (g/cm3). That's our starting point; we'll compare other things to this density to figure out why they sink or float.
This is where oil comes into play. Oil, whether used in cooking or found in nature, is lighter than water. Its density is typically less than 1 g/cm3, making it less dense with molecules than water.
Consider oil to be a lightweight superhero, and water to be a slightly heavier sidekick. Because oil is less dense than water, it prefers to float. It's similar to dropping a handful of popcorn into a bowl of water; the popcorn floats because it's less dense.
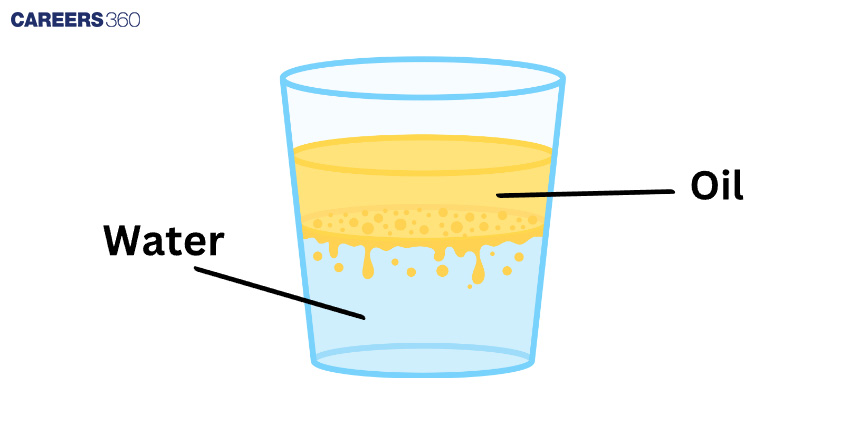
Look at the image: there's a glass of water with oil on top. The oil remains on top because it is lighter than water, similar to a feather on top of a rock. Water is like heavy stuff, while oil is like light stuff. Because it is light, the oil floats to the top. As a result, in the image, the oil is floating on the water. It happens due to "buoyancy," a special force that causes things to float. It's like playing with toys in the bathtub; some float, while others sink. Because it is light, oil prefers to float to the top.
Density Stratification
Now that we've understood density, let's talk about "density stratification." It sounds complicated, but it's similar to layering things based on how heavy or light they are. Consider stacking your toys, lighter ones on top and heavier ones on the bottom. That's a bit like density stratification.
Consider a glass of water with oil on top, as shown in the image. This is also true in large bodies of water, such as oceans or lakes. When something less dense, such as oil, collides with something denser, such as water, layers form. The lighter oil floats to the surface, forming a sort of "floating layer," thanks to the magic of buoyancy.
Let's apply this to real life. You've probably heard about oil spills in the oceans or lakes. Because oil is less dense than water, it forms a layer on the surface when it enters the water. This can be harmful to fish and other sea creatures.
Consider it like spilled juice on your table: the juice floats to the top because it is lighter, and the table represents the heavier water below.Understanding density stratification helps scientists and people figure out how to clean up these spills and take care of our water homes better.
Archimedes' Principle in Action
Remember Archimedes, the brilliant scientist we discussed earlier? His principle is the superpower that explains why things float or sink in water. So, how does it work in the case of oil? When something is placed in water, it pushes the water aside. According to Archimedes' principle, water pushes back with a force known as buoyant force.
Consider a tiny oil droplet floating in water. Gravity wants to pull it down, similar to how when you drop a ball, it falls to the ground. But this is where buoyancy comes in handy. The buoyant force of the water pushes up on the oil droplet.
It's similar to having two tug-of-war teams. On one hand, gravity wants to pull the oil down, while buoyancy wants to push it up. When the buoyant force is greater, the oil floats, much like a balloon that wants to rise. So, in our glass of oily water, there's a secret battle going on between gravity and buoyancy. Buoyancy wins, keeping the oil above the water.
And that's why oil floats on water, like a special trick by nature! Knowing this helps us take care of our water and keep it clean and safe.
Also Read- Learn To Solve Maths Alligation And Mixtures Problems like A Pro
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
