JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
In this article we will have organometallic compound notes, organometallic compound pdf, and organometallic chemistry questions and answers pdf.
Organometallic compounds definition is that as those chemical species in which the central atoms are bonded directly to one or more carbon atoms of organic functional groups. The term metal generally stands for elements which are less electronegative than carbon. Examples are boron, silicon etc. The bonding interactions in organometallics compounds should be ionic or covalent, localized or delocalized between one or more carbon atoms present in an organic group or molecule and a transition, lanthanide, actinide or main group metal atoms. Organometallic reagents are those compounds that consist of carbon-metal bonds. Organometallic compounds examples: (C2H5)4Pb, (CH3)3SnH, ferrocene etc.
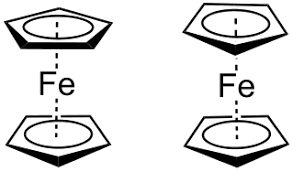
Organometallic chemistry is viewed as a bridge between organic and inorganic chemistry. Many organometallic compounds shows fluxionality. An example of fluxional organometallic compound is Fe(η5-C5H5) (η1- C5H5)(CO)2.Organo meaning in hindi is ‘ang’.
Also read -
Hapticity of the ligand is the number of carbon atoms through which a ligand is attached. It is represented by the symbol ‘ηn’ where n is the number of carbon atoms of the ligand attached with the metal. Based on the number of ligands attached Hapticity can be classified as follows:
Monohapto ligands (η1):
The organic ligands which are attached with metal through one carbon atom only are monohapto ligands.
Eg. C2H5-Mg-X, CH3-M
Dihapto ligands (η2):
The ligands which are attached to metal through two carbon atoms are called dihapto ligands.
Eg. K [Pt Cl3 (CH2=CH2)
Trihapto ligands (η3):
The ligands which are attached to metal through three carbon atoms are called trihapto ligands.
Eg. Allyl groups
Tetrahapto ligands (η4):
The ligands that are attached to metal through four carbon atoms are called tetrahapto ligands.
Eg. Butadiene, cyclobutadiene etc.
Likewise, we can say that cyclopentadienyl anion, benzene, cyclo-octatetraene are pentahapto, hexahapto, octahapto ligands respectively.
Most of the organometallic compounds are coordination compounds that consist of one or more ligands attached to the metal M through M-C bonds. The rules governing their nomenclature are therefore similar to those naming of coordination compounds. In the case of organometallic compounds few additional rules are observed.
The ligands (without the prefixes mono, di, tri etc.) are named in alphabetical order irrespective of the charge present on them.
The presence of hydrocarbon ligands in the coordination sphere is indicated by the names of their radicals (methyl, ethyl etc.).
In case of unsaturated molecules or groups, the ligand is named with the prefix ηn.
Organometallic compounds can be classified as follows:
Ionic organometallic compounds
Most of the organometallic compounds of alkali metals (with exception of lithium compounds which are largely covalent) fall in this category.
Such compounds are colourless salt like solids and are soluble in polar solvents.
They are quite reactive and are kinetically unstable.
Mostly ionic organometallic compounds have a short life.
Sigma (σ) bonded organometallic compounds
Metallic elements of group 2,13,14,15 as well as the transition metals with fully filled d-orbitals such as Zn, Cd and Hg produces organometallic compounds where the bonding of metal atoms to carbon atoms occurs via sigma (σ) bond.
Multicentered organometallic compounds
Organometallic compounds which are loosely bound are called electron deficient and occur in polymeric forms that fall under this category. These compounds can be regarded as a halfway between the σ bonded organometallic compounds of Sn, Pb etc. and ionic organometallic compounds of alkali metals. Elements which have a high tendency to form multicentre organometallic compounds are Li, Br, Mg, B and Al.
Pi (π) bonded organometallic compounds
These include organometallic compounds of alkene, alkyne and other carbon-containing compounds which have electrons in their π - molecular orbital. Combination of these π-molecular orbitals with the vacant orbitals of metal atoms results in a pattern where the metal atom gets bound to all the carbon atoms which is spread by organo ligands on the π - molecular orbitals.
Eg. Ferrocene
Direct method
Aromatic compounds directly reacts with alkali metal.
Eg. C10H8 + Na → Na (C10H8)
Reaction with alkyl halide metal
Magnesium reacts with alkyl or aryl halide in ether to give Grignard reagent which is an organometallic compound.
Eg. CH3Cl + Mg ether CH3MgCl
Transmetallation method
It involves the replacement of one metal by another. It is achieved by the action of more electropositive metal such as zinc with a compound having metal with low electropositivity.
Eg. (CH3)2 Hg + Zn → (CH3)2 Zn + Hg
Due to the lanthanide contraction property of mercury (Hg), it becomes more electropositive than zinc (Zn).
Double decomposition method
In such a reaction, the alkyl group migrates to the more electronegative metal.
Eg. Li4 (CH3)4 + SnCl4 → 4 LiCl + Sn (CH3)4
Tin (Sn) is more electronegative than lithium (Li).
Oxidation addition reactions
These are the additional reactions which are accompanied by oxidation.
Eg. RhCl [P(Ph3)]3 + CH3I → RhCl I (CH3)(PPh3)2 + PPh3
Also, students can refer,
The properties of organometallic compounds are given below:
The carbon atom and the metal are usually bonded through covalent bonds.
Mostly when the compounds have aromatic hydrocarbon groups or a ring structure, the organometallic compound exists in solid states.
Volatile organometallic compounds are found toxic to humans.
They act as reducing agents.
Organometallic compounds have many applications in the field of chemistry. Some of them are listed below:
Organometallic compounds are used as homogeneous catalysts in certain commercial chemical reactions.
They can be used as stoichiometric reagents for both research-oriented and industrial chemical reactions.
These compounds are used for the production of some semiconductors, which is in necessary of compounds like trimethylgallium, trimethylaluminum, trimethylindium, and trimethyl antimony.
They are used for production of light emitting diodes (or LEDs).
These compounds are used as catalysts and reagents for the synthesis of some organic compounds.
Many organic compounds are synthesised from the complexes formed from organometallic compounds.
Organolithium compounds are organometallic compounds that consist of carbon-lithium bonds. These compounds are of great importance. The use of Organolithium compounds are widely for chemical research and industrial application. The reactivity of these compounds resembles Grignard reagents, but are more reactive.
Reaction of aryl or alkyl chloride with lithium metal in presence of benzene or an aliphatic hydrocarbon.
RCl + 2Li → RLi + LiCl
Reaction via metal-hydrogen exchange, metal-halogen exchange, and metal-metal exchange
RH + R′Li → R′H + RLi
The structure of organolithium compounds have high oligomeric nature due to the presence of 3-center 2-electron bridging bonds. The extent of oligomerization depends on the alkyl or aryl group present.
They are low melting solids or liquids.
They have high volatility due to the covalent bonding.
They are soluble in ethers, aliphatic and aromatic compounds.
They rapidly react with air and water.
The reaction with water is the foundation of Gillman double titration method which determines the concentration of organolithium reagents in solution.
Also check-
NCERT Chemistry Notes:
Tetramethyl aluminium
Tetraethyl lead
Ziese’s salt
Cis-Platin
Answer: d) Cis-platin
The chemical formula of cis-platin is [PtCl2(NH3)2][PtCl2(NH3)2]. Even though this compound has a metal it lacks a carbon atom. Hence it is not an organometallic compound.
Sodium ethoxide
Ethyl magnesium bromide
Ferrocene
Grignard reagent
Answer: d) sodium ethoxide
Organometallic compounds are those compounds in which carbon atoms and metal are directly bonded. But in sodium ethoxide oxygen is attached to sodium metal so it is not an organometallic compound.
Organometallic compounds can be defined as those chemical species in which the central atoms are bonded directly to one or more carbon atoms of organic functional groups.
a) Cis-Platin
b) Ferrocene
c) Sodium ethoxide
Answer: b) Ferrocene
Hapticity is the number of carbon atoms through which a ligand is attached. It is represented by the symbol ‘ηn’ where n is the number of carbon atoms of the ligand attached with the metal.
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC
Apr 27, 2022 - 12:42 p.m. IST ---STATIC

As per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

Get up to 90% scholarship on NEET, JEE & Foundation courses

As per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

Enrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

Start your JEE preparation with ALLEN

Ace your NEET preparation with ALLEN Online Programs