Understanding The Science Behind Melting Of Ice
On a hot summer day, imagine a popsicle. The solid ice gradually melts and transforms into a wet puddle as the sun beams on it. It appears to be magic, but remember there is science at play. Ice is made of microscopic fragments that enjoy holding hands, or, in scientific terminology, forming "molecular bonds." These linkages keep the ice firm and frozen. When we add heat, such as heat from the sun or your fingers, those little bits become activated, start moving, and eventually separate.
This Story also Contains
- Molecular Structure of Ice
- Absorption of Heat Energy
- Temperature Change
- Overcoming Intermolecular Forces

This transformation from ice to water is critical to many things in our environment. It is the reason we can drink cold drinks on a hot day and why ice cubes melt in a glass of water. But there's more to it than that. Understanding how and why ice melts is particularly important for scientists studying climate change, as well as for everyday circumstances such as creating ice cream and keeping our roads safe in the winter. So, let's delve into the mysterious physics of ice melting and discover its fascinating real-world applications!
Molecular Structure of Ice
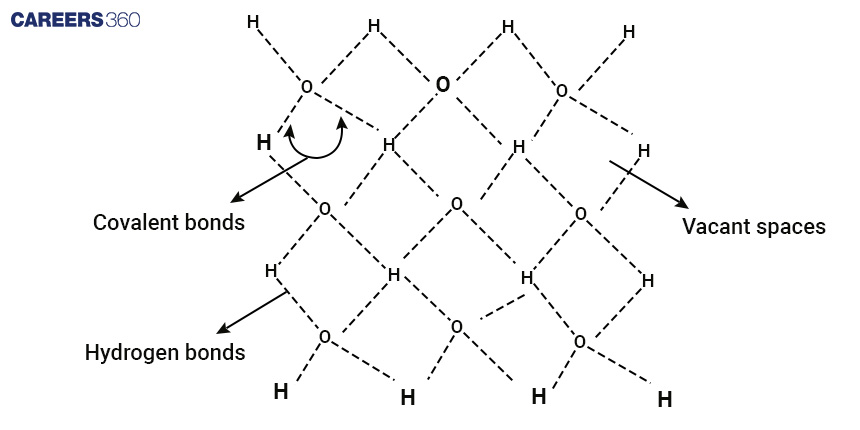
Ice has a particular molecular structure that distinguishes it from its liquid counterpart, water. Water molecules are made up of two hydrogen atoms and one oxygen atom that are bonded together by covalent bonds. The important aspect of ice is the arrangement of water molecules. Ice solidifies into a hexagonal lattice structure, similar to a three-dimensional grid. This systematic design produces uniform spacing and a repeating pattern. The significance of hydrogen bonding, however, is crucial in understanding why ice remains solid at low temperatures.
The importance of hydrogen bonding in the firmness of ice cannot be overstated. These bonds are relatively strong in comparison to other intermolecular forces, and they are caused by electrostatic attractions between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another. In the case of ice, hydrogen bonds behave as tiny, resilient springs, securely holding the water molecules in place within the lattice structure. They give structural stability by resisting molecular mobility. This network of hydrogen bonds forms a stiff, organised structure that makes moving water molecules difficult.
Therefore, ice remains solid at temperatures below its melting point. To begin the transition from solid ice to liquid water, sufficient heat energy must be supplied to disrupt these hydrogen bonds, allowing the water molecules to move more freely and, as a result, melting the ice.
Absorption of Heat Energy
Heat, which is a type of energy, is essential in the melting of ice. This energy is exchanged between objects due to temperature variations, and it is linked to the kinetic energy of particles such as atoms and molecules at the microscopic level. When these particles move faster, their kinetic energy increases, resulting in an increase in temperature. Heat energy is measured in measures such as calories or joules and is responsible for a wide range of physical and chemical processes.
When this heat energy is absorbed by the ice, it melts. This energy can come from a variety of natural and man-made sources. The Sun, which radiates heat through space, warming the Earth's surface, and geothermal heat from beneath the Earth's core are two natural sources. Human-made sources include a wide range of technology, from fuel-burning combustion engines to electrical heating components and chemical reactions. These sources create heat for a variety of uses, including heating our homes and generating power. Ice absorbs thermal energy when it is exposed to an external heat source, such as a hob or the Sun's rays. This raises the temperature of the ice, which starts the melting process, changing the solid ice into liquid ice.
Temperature Change
When heat is applied to ice, it absorbs the energy and raises its temperature. This rise in temperature is what causes the ice to melt. The melting point is the precise temperature at which this transition from solid ice to liquid water occurs. The melting point of pure ice at standard atmospheric pressure is precisely 0 degrees Celsius (32 degrees Fahrenheit). At this temperature, the ice's molecular structure begins to degrade, allowing water molecules to flow more freely and resulting in the transition from solid to liquid.
Overcoming Intermolecular Forces
Intermolecular forces are crucial in understanding why ice melts. These forces keep ice solid, with hydrogen bonds being the major intermolecular interactions in this case. These hydrogen bonds arise as a result of interactions between positively charged hydrogen atoms in one water molecule and negatively charged oxygen atoms in other surrounding water molecules, resulting in the formation of a stable lattice structure in ice.
When heat energy is delivered into ice, the temperature rises, which causes an increase in molecular mobility. This is because temperature is a measure of the average kinetic energy of a substance's molecules. As the ice absorbs heat energy, its molecules move more furiously, demonstrating increased kinetic energy.
This increased molecular motion has a significant impact on the hydrogen bonds within the ice. Because of the higher kinetic energy, the water molecules are able to break free from their fixed places in the ice lattice, weakening the hydrogen bonds that hold them in place. This weakening allows water molecules to travel more easily past each other, resulting in the transition from the solid state of ice to the liquid state of water.
And that's why ice melts when it gets warm. It's like the ice is made of tiny building blocks, and when it gets hot, these blocks start to move around and fall apart, turning into water. This helps us enjoy cold treats on a hot day and is important for many other things, like understanding the weather and making yummy ice cream. So, the next time you see a popsicle melting, you'll know it's not magic but a cool science lesson!
Also Read- Mysteries Of A Rainbows: How Does Sunlight Transform Into Colourful Arcs?
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
