LED Wonders: The Art of Crafting Different Colours
Have you ever found yourself mesmerised by the vibrant hues emitted from the tiny bulbs in your electronic gadgets, toys, or even your room's lighting? Those captivating colours aren't just random; they are the result of a fascinating process happening inside Light-Emitting Diodes, or as we commonly know them, LEDs.
This Story also Contains
- The Basics of LED Structure
- Meet the Materials - Colors and Semiconductors
- The Science of Colour Emission
- Creating a Rainbow - Colour Mixing in LEDs
- The White Light Mystery

Imagine this: a world without the red glow of your TV's standby light, the green brilliance that signals it’s okay to move ahead on the road, or the soothing white illumination in your room. LEDs have become an integral part of our daily lives, adding a splash of colour and brightness to our surroundings. But have you ever wondered how these magical lights create such a dazzling array of colours? Let's take a closer look at LED technology and understand the secrets behind the colours that captivate our eyes and bring the world to life.
The Basics of LED Structure
A Light Emitting Diode (LED) operates on a semiconductor structure characterised primarily by the P N Junction. The positive side is formed by the p-type semiconductor, which is frequently gallium or aluminium-doped gallium arsenide or phosphide, while the negative side is formed by the n-type semiconductor, which is commonly made of materials such as silicon or gallium nitride. When these materials come into contact, a p-n junction forms, forming a depletion zone that prevents further electron flow. When a forward voltage is applied across the junction, electrons and holes migrate, resulting in recombination and the release of energy in the form of photons, which produces the characteristic light emission in LEDs.
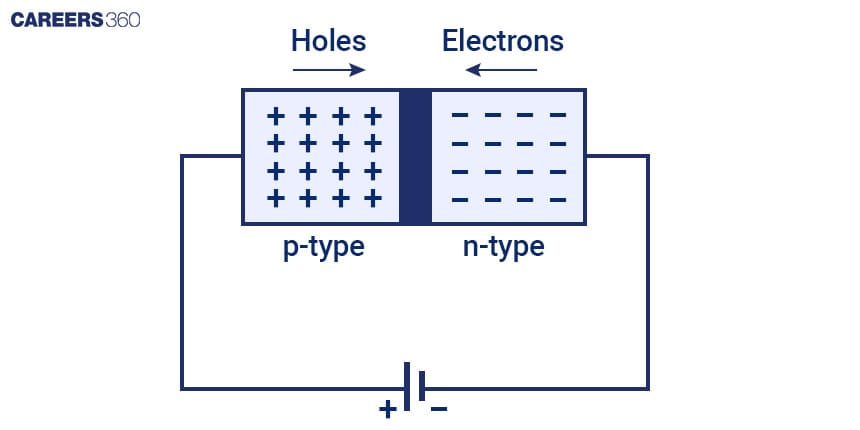
The semiconductor materials used in LED construction are critical in determining the colour of the emitted light. The band gap energies of various materials, such as gallium arsenide, gallium phosphide, gallium nitride, indium gallium nitride, and aluminium indium gallium phosphide, are chosen. The energy difference between the valence and conduction bands, represented by the band gap, is critical for determining the colour of light emitted by an LED. Low band gap materials, for example, produce red LEDs, medium band gap materials produce green LEDs, and high band gap materials produce blue LEDs. Engineers can tailor the band gap to design LEDs for specific applications ranging from lighting and displays to various electronic devices.
Meet the Materials - Colors and Semiconductors
LEDs' vibrant red colours are typically caused by aluminium gallium arsenide (AlGaAs) or gallium arsenide phosphide (GaAsP). These materials have specific band gap energies that, when stimulated by an applied voltage, cause photons of the red wavelength to be released. Red LEDs play an important role in providing visual cues in our daily lives, whether it's the standby indicator on your TV or the tail lights on a vehicle.
Green LEDs rely on semiconductor materials such as aluminium gallium phosphide (AlGaP) or gallium phosphide (GaP) to produce their soothing and lively glow. These materials have band gap energies that cause light to be emitted in the green part of the spectrum. Green LEDs are used in a wide range of applications, from traffic lights to electronic devices, and they combine functionality and aesthetics.
Blue and white LEDs, which provide cool tones to our surroundings, make use of semiconductor materials such as gallium nitride (GaN) or indium gallium nitride (InGaN). Because these materials have higher band gap energies, they can produce shorter-wavelength light. Blue LED development was a significant breakthrough because it paved the way for the creation of white light by combining blue LEDs with phosphors.
Technologists meticulously selects semiconductor materials based on their band gap energies to customise LED characteristics.They can precisely design LEDs for specific applications by manipulating the composition of materials. LEDs' versatility has led to their widespread use in a wide range of applications, from lighting and displays to cutting-edge electronic devices.
The Science of Colour Emission
The concept of the band gap—a critical factor determining the colour of light emitted—is at the heart of LED technology. The band gap is the difference in energy between the valence and conduction bands in a semiconductor material. Each material used in LED construction has a different band gap energy, which influences the colour of the emitted light.
When a forward voltage is applied across the p-n junction of the LED, electrons in the conduction band gain enough energy to move to the valence band. This electron-hole transition (electron vacancies in the valence band) results in a process known as recombination. Electrons falling back into holes release energy in the form of photons during recombination. The energy of these photons corresponds to the semiconductor material's band gap energy, which defines the colour of the emitted light.
The energy gained while moving across the p-n junction is released in the form of light as electrons and holes recombine in the semiconductor material. This process is consistent with the basic principle that when electrons return to a lower energy state, they emit energy in the form of electromagnetic radiation—photons in the case of LEDs.
The wavelength of the emitted light corresponds to the energy difference between the valence and conduction bands, which is determined by the semiconductor material's band gap. Longer wavelengths (red light) are produced by lower band gap energies, while shorter wavelengths (blue light) are produced by higher band gap energies.
Also check - Geometry In High School: Real Life Applications Of Various Shapes
Creating a Rainbow - Colour Mixing in LEDs
Technologists use the concept of colour mixing to unleash the full spectrum of colours and create a visual feast by combining different semiconductor materials in a single LED package. The most common approach involves combining red, green, and blue LEDs into a single unit, giving rise to RGB LEDs.
Colours are created by combining different light sources in additive colour mixing. The additive colour model in the case of LEDs involves combining light emitted from red, green, and blue sources. By varying the intensity of each colour, a wide spectrum, including white light, can be created.
LED displays, such as those found in televisions, monitors, and large digital screens, use additive colour mixing to produce a wide range of colours. In these displays, each pixel is made up of red, green, and blue subpixels. The display can reproduce a full spectrum of colours by varying the intensity of each subpixel. When compared to other methods, this method provides a more dynamic and vivid colour representation.
The White Light Mystery
White LEDs have become a fixture in our lighting landscape, offering a versatile and energy-efficient alternative to traditional light sources. Rather than simply combining red, green, and blue LEDs to produce white light, the process of producing white LEDs involves a fascinating phenomenon known as phosphor conversion.
White LEDs are frequently formed from blue LEDs, though ultraviolet LEDs are also used. Blue LEDs have a higher energy level, making them suitable for the phosphor conversion process that follows.
The blue or ultraviolet LED is coated with phosphor material. Phosphors are substances that absorb and re-emit light energy at a longer wavelength. The phosphor coating in white LEDs absorbs blue or ultraviolet light and re-emits it across a broader spectrum, including longer wavelengths associated with white light.
When the original blue or ultraviolet light emitted by the LED is combined with the light re-emitted by the phosphor coating, the result is a mixture that appears as white light. The phosphor material's specific composition influences the quality and characteristics of the resulting white light, allowing for fine-tuning to meet a variety of lighting requirements.
Discovering how LEDs work helps you appreciate the colours in your gadgets and lights. Now, you can make smart choices for energy-efficient lighting and understand the tech brightening up your world.
Also check - Power Up Your Knowledge: Explore The World Of Transformers
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
