Chemical Effect Of Electric Current On Different Materials: Class 8 Physics
Can the colour of a liquid change if electricity is passed through it? Yes, but what colour you see will depend on the specific characteristics of the liquid and the electrical current being applied. Some liquids may start emitting light due to electrochromism, the process by which ions in the liquid become excited and emit light when an electric current is passed through the solution.
Electroplating of metals, the production of chlorine and caustic soda through the electrolysis of salt water, and the production of aluminium from aluminium oxide; these are all examples of chemical reactions caused by the flow of electricity through a material. In each of these cases, the electric current drives a chemical reaction that would not otherwise occur, allowing for the production of various materials and chemicals.
Here’s more on how electricity flows, the chemical effects of an electric current and electroplating.
Conductors, Insulators: A Quick Introduction
These are the materials required for electricity to flow :

First, you need an energy source, a battery or a cell. Now energy has to be transmitted from one place to another, for which a path through which energy can pass is required. The wires form our transmission system. Now, we need to check if the current is flowing through the circuit. For that, we need an indicator. The indicator can be a blub, fan or horn.
Can electricity flow through every material?
Electricity is the flow of electrons through a conductor. In order for electricity to pass through a material, it must have a sufficient number of free electrons that are able to move and carry the current. Materials with a large number of free electrons tend to have high electrical conductivity and are good conductors of electricity.
Some common examples of good conductors of electricity include metals such as copper, aluminium and gold. These materials are good conductors because they have a high number of free electrons that are able to move freely through the material, allowing electrical current to flow easily.
Copper is often used in electrical wiring due to its excellent conductivity and its ability to resist corrosion. Aluminium is also a good conductor of electricity and is often used in power transmission lines and in the construction of aircraft due to its low density and strength. Gold is a very good conductor of electricity and is often used in electronic circuits and in the production of connectors and switches due to its high resistance to corrosion.
The electrical conductivity of a material is determined by its atomic structure and the arrangement of its atoms. Materials with metallic bonds, where the electrons are shared between atoms, tend to have high electrical conductivity because the electrons are free to move and carry the current. Materials with covalent bonds, where the electrons are tightly bound to the atoms, tend to have low electrical conductivity because the electrons are not as free.
Glass has very low electrical conductivity and is not a good conductor of electricity. Glass is made up of silicon dioxide(sio2) molecules, which are held together by a covalent bond.
How does electricity flow?
Electricity is the flow of electrons through a conductor. In order for electricity to flow, there must be a circuit, which is a closed loop of conductive material through which the electrons can flow.
The flow of electricity begins at a power source, such as a battery or generator. The power source generates a voltage difference, or potential difference, which causes the electrons to flow from a region of higher potential energy to a region of lower potential energy.
The flow of electrons is facilitated by the conductor, which is a material that allows the electrons to move freely through it. The conductor is connected to a load, which is a device that uses electricity, such as a light bulb or an appliance.
As the electrons flow through the circuit, they encounter resistance, which is a measure of how difficult it is for the electrons to flow through the conductor. The resistance of the conductor, along with the voltage of the power source, determines the amount of current that flows through the circuit.
The flow of electricity involves the movement of electrons through a circuit, facilitated by a conductor and powered by a voltage difference. The resistance of the conductor and the load determine the amount of current that flows through the circuit.
What are good insulators?
A good insulator is a material that does not conduct electricity well and has a high resistance to the flow of electric current. Good insulators are used in a variety of applications, including electrical wiring, electronics, and construction.
Materials such as rubber, glass, and plastic, which have a low density of free electrons and do not conduct electricity well, are often used in electrical insulation. Insulating materials help prevent electrical current from flowing through unintended paths or causing short circuits.
The conductivity of a material can depend on various factors, such as its temperature, and the presence of impurities or defects in its structure. Some materials may also exhibit different conductive properties depending on the type of energy they are conducting – electrical, thermal, or others.
Chemical Effect Of Electric Current
Electricity passing through a substance causes chemical reactions; this is the chemical effect of electric current. These reactions can be caused by the flow of electrons through the substance and can result in a variety of chemical changes.
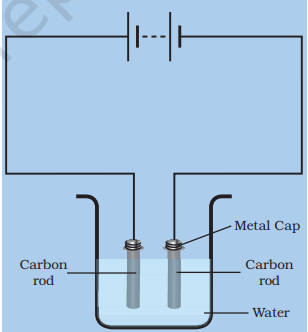
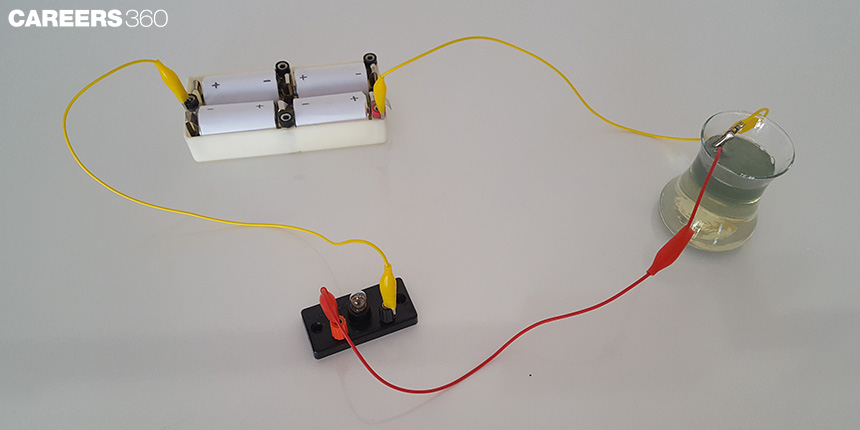
Let’s understand the chemical effect of electric current with the help of the above experiment.
At home, when an ordinary cell gets damaged, you can extract a carbon rod from it and rub the metal cap on it to remove any rust. You can take two such carbon rods. A metal rod is connected to a small battery with wire. When the circuit is closed, current will flow through the wires causing a chemical reaction that will lead to a bubble forming on the metal cap. The current splits the water molecules into hydrogen and oxygen which bubbles out. The process is called electrolysis.
Chemical Effect Of Electric Current: Examples
Here are some common examples:
Electrolysis: This is the process of using electricity to break down a substance into its component ions. Electrolysis is commonly used to produce a variety of chemicals, including chlorine, sodium hydroxide, and hydrogen gas.
To produce hydrogen gas through electrolysis, an electric current is passed through water (H2O), which splits the water molecules into hydrogen and oxygen gas. The oxygen is released into the air, while the hydrogen gas is collected for use.
Electroplating: This is the process of using electricity to coat a metal object with a layer of a different metal. Electroplating is commonly used to improve the corrosion resistance, appearance, or electrical conductivity of a metal object.
An example of electroplating is the chrome plating of car parts, Which is generally made of zinc and is cleaned and polished to remove any dirt or imperfections. It is then submerged in an electrolyte solution containing chromium ions. An electric current is passed through the solution, causing the chromium ions to be deposited onto the surface of the car part. This process is repeated until a sufficiently thick layer of chromium has been deposited.
Other examples of electroplating include the production of jewellery, the coating of electronic components, and the production of decorative items such as vases and figurines.
Corrosion: These are chemical reactions that cause a material to break down and deteriorate. When electricity passes through a metal conductor, it can corrode the conductor if it is simultaneously exposed to a corrosive environment, such as salt water.
The most familiar example of corrosion due to electricity is galvanic corrosion, which occurs when two different metals are electrically connected in the presence of an electrolyte, such as saltwater. The two metals form a galvanic cell, and an electric current flows between them, causing one metal to corrode faster than it would otherwise. This type of corrosion is often seen in metal structures such as bridges and ships, where different metals are used in close proximity and are exposed to saltwater.
Chemical reactions: Electricity can also cause chemical reactions that generate heat or light. For example, When electricity passes through a light bulb, it causes resistance in the filament, which is a thin wire made of a material such as tungsten. This resistance causes the filament to heat up, and as it gets hotter, it begins to glow. The glowing is due to the release of energy in the form of light as a result of the movement of electrons within the atoms of the filament material.
Electroplating
Electroplating is a process in which a thin layer of one metal is deposited onto the surface of another metal using an electric current. This is achieved by immersing the object to be plated (the "substrate") in a solution containing the metal ions that will be used to form the plating layer (the "plating metal"). An electric current is then passed through the solution, using the substrate as one electrode and the plating metal as a separate "counter electrode".
As the electric current flows through the solution, the metal ions in the solution are attracted to the substrate and are deposited onto its surface. This process results in the transfer of metal atoms from the solution to the substrate, forming a thin layer of metal on the surface of the substrate. The thickness and composition of the plating layer can be controlled by adjusting the concentration of the metal ions in the solution and the duration of the electroplating process.
Electroplating is commonly used for a variety of purposes, including to improve the appearance and corrosion resistance of metal objects, to increase the conductivity of a material, and to provide a protective layer on a surface. It is also used in the production of electronic components, such as circuit boards and connectors.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters