How Many Tetrahedral Voids are Occupied in Diamond
Voids are the voids that are present in crystal formations. These gaps can emerge because of the various atom arrangements. Four spheres are placed in a tetrahedral configuration, and spaces between them contain tetrahedral voids. This void forms when a sphere from the second layer is placed over the void of the first layer. In a crystal, a single triangular void or space is surrounded by four atomic spheres. The tetrahedral vacancy consequently has a coordination number of four.
Diamonds have a ZnS-type structure. Carbon atoms occupy all of the fcc lattice locations as well as alternate tetrahedral vacancies. Half of the tetrahedral gaps in the diamond are filled. The diamond's carbon fills four of the eight tetrahedral voids in the fcc unit cell. As a result, a diamond has 4 tetrahedral voids.
Structure of Diamond
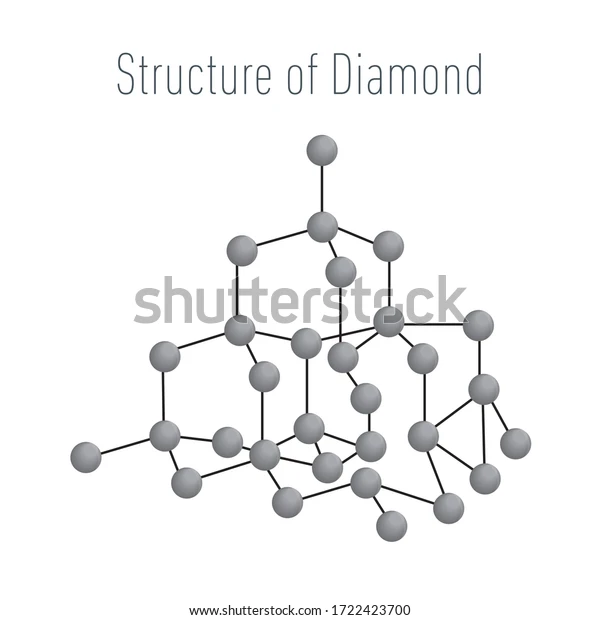
It is claimed that all four carbon atoms in a diamond have strong chemical connections with one another, creating a perfect tetrahedron structure that extends throughout the crystal. Here, the carbon atoms are sp3 hybridised, and their bonds to one another are of identical length. Diamond subsequently creates a three-dimensional network of potent covalent connections.
The melting point of the diamond is around 3843 K, and its density is approximately 3.51 g/cm3. Since its valence electrons form C-C sigma covalent bonds and are so confined and unable to conduct electricity, it is known to be a poor conductor of electricity.
Diamond Optical Characteristics
Diamond is an isotropic crystal that ranges in transparency from translucent to opaque. Diamonds have four primary optical properties: optical absorption, lustre, fluorescence, and colour.
Diamonds exhibit fluorescence, which produces light with various colours and fervour when exposed to long-wave UV radiation (365 nm).
Optically, some diamonds show a notable absorption spectrum with a thin line in the violet at 415.5 nm, however, this line is typically undetectable until the diamond has been cooled to room temperature.
Lustre: A diamond's shine is described as "adamantine," which implies diamond-like. Because a diamond's sides are flat, reflections on them are undistorted. A diamond has a refractive index of 2.417. Diamond's cubic structure makes it isotropic. Cut diamonds' palpable fire is a manifestation of its high dispersion of 0.044.
Diamonds come in a variety of hues, including brown, yellow, grey, black, white, blue, orange, and red. Crystallographic flaws, including structural flaws and colour-inducing substitutional impurities, are present in coloured diamonds. Pure diamonds should theoretically be transparent and colourless. There are two diamond classes, according to science:
The main impurity in type I diamonds, with a concentration of up to 1%, is nitrogen (N) atoms. The N atoms have no impact on the colour of the diamond if they are arranged in larger groups or pairs. Nitrogen impurities are substantially less prevalent in type II diamonds.
Diamond's Physical Characteristics
The strong carbon-carbon covalent connections must be broken throughout the structure before melting can take place, giving the diamond a very high melting point.
Diamond is incredibly tough.
It is not an electrical conductor.
Both water and organic solvents cannot dissolve them.
Materials' Qualities of Diamond
Both the presence of boron ( 20 ppm), which is responsible for Type IIb diamond's semiconducting characteristics and nitrogen, which is a prominent impurity in diamonds, have been noted. Although 58 elements have been identified as trace impurities, diamond is just a moderately effective chemical sink for a variety of elements in terms of concentration levels and colour of diamonds
The source of the brown and colour in diamonds is nitrogen, which is by far the most frequent contaminant discovered in gem diamonds.
The yellow diamond is the rarest of all diamond colours, and it is followed by brown, colourless, blue, green, black, pink, orange, purple, and red.
While pure or almost pure diamonds are transparent and colourless, coloured diamonds have impurities or structural flaws that give them their colour.
In a method that roughly mimics the circumstances in the Earth's mantle, high pressure and high temperature can also be used to create diamonds artificially. Chemical vapour deposition is an alternative and wholly distinct growth method (CVD).
Applications
Diamonds have a wide range of uses because of their excellent physical characteristics.
Its suitability for daily use due to its scratch resistance, unlike many other stones, may have contributed to its appeal as the preferred diamond in engagement or wedding rings, which are typically worn every day.
The diamond is the most important gem in the jewellery industry.
Although it is common to use yellow and brown stones, the colourless stone is the most preferred option for jewellery.
Apertures in lasers, x-ray equipment, and vacuum chambers are covered with diamond windows. They are produced using thin membranes made of diamond.
Frequently Asked Questions (FAQs)
It is incorporated into audio equipment to enhance sound quality. Diamonds are hard and easily vibrate at fast speeds, which results in high-quality sound. High-end recorders and DJ equipment both use it. It serves as diamond record needles in these. Potential health advantages of nanodiamonds.
The world's strongest natural substance is a diamond.
Diamonds are created and brought to the surface by volcanic activity at a depth of about 100 miles.
Diamonds are the only gems produced from a single element.
To get right to the point, diamonds cannot be destroyed. They are the world's hardest mineral, nevertheless. The Greek word "Adamas," which means "unconquerable and indestructible," is where the term "diamond" originates. But they are susceptible to harm and even destruction, just like everything else in this world.
In addition to these negative effects, diamond mining also destroys ecosystems and causes soil erosion and deforestation. Blood diamonds are a significant political impact on the diamond commodity chain, particularly at the mining level. These diamonds are created in conflict areas to fund civil wars.
The carbon atoms in a diamond are organised in a diamond cubic crystal lattice, making it an allotrope of carbon. Diamond is the material with the highest heat conductivity and hardness among all naturally occurring substances.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
