How Many Structural Isomers are Possible for Butane
Introduction
There are two structural isomers possible for butane. A structural isomer of a compound is another compound whose molecules have the same number of atoms of each element but with different bonds between them. The atoms are arranged in a different order with the same molecular formulas. Structural isomerism or constitutional isomerism is a kind of isomerism in which the molecules have the same molecular formula with different orders and bonds.
Structural Isomers of Butane
Butane is an alkane that is made up of four carbon atoms. Alkanes are the simplest forms of hydrocarbons with all C-C bonds. They are also known as saturated hydrocarbons. Their general formula is C_{n}H_{2n+2} ![]() . The formula of Butane is hence C_{4}H_{10}
. The formula of Butane is hence C_{4}H_{10} ![]() . Butane exhibits structural isomerism. There are two possible structural isomers of butane which are:
. Butane exhibits structural isomerism. There are two possible structural isomers of butane which are:
n-Butane
Isobutane
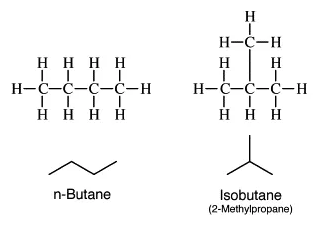
n-Butane is a straight-chain four-carbon compound in which the atoms are bonded with single covalent bonds. The formula of n-Butane is the same as Butane which is C_{4}H_{10} ![]() .
.
The structure of n-Butane is depicted as CH_{3}-CH_{2}-CH_{2}-CH_{3} ![]() .
.
Isobutane on the other hand is also a four-carbon compound but it has a different structure. In isobutane, three carbon atoms form the parent chain while one carbon atom is placed as the side chain at the second carbon of the parent chain. The formula of isobutane is also the same as Butane which is C_{4}H_{10} ![]() . The IUPAC nomenclature of isobutane is 2-methylpropane. The structure of isobutane is depicted as CH_{3}-CH(CH_{3})-CH_{3}
. The IUPAC nomenclature of isobutane is 2-methylpropane. The structure of isobutane is depicted as CH_{3}-CH(CH_{3})-CH_{3} ![]() .
.
Conclusion
There are two structural isomers possible for butane. These are n-Butane and isobutane. Both of these isomers have the molecular formula C_{4}H_{10} ![]() . They have the same molecular formula but differ in their structure.
. They have the same molecular formula but differ in their structure.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
