How Many Sigma and Pi Bonds are Present in Toluene
Introduction
There are 3 pi bonds and 15 sigma bonds in toluene. Toluene goes by the formula C6H5CH3. It is an aromatic hydrocarbon. Toluene is a mono-substituted benzene derivative consisting of a methyl group. Toluene is a volatile liquid which turns into vapour at room temperature. It is an essential organic solvent in the chemistry lab as most of the compounds are soluble in toluene. The Sigma bond is the strongest covalent bond which is formed by head-on overlapping of orbitals. The Sigma bond is the first bond formed between two atoms.
On the other hand, pi bonds are covalent bonds characterised by the lateral overlap of the two lobes of the orbital with that of another atom's orbitals. Pi bonds are comparatively weaker bonds than sigma bonds. 2 electrons form one bond. The electrons of the pi bonds are delocalised electrons. They are responsible for the aromaticity of the compound.
Explanation
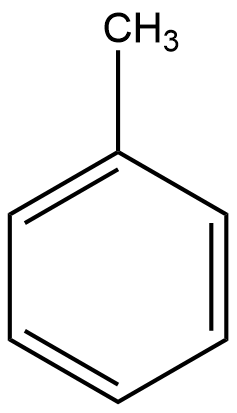
This is the chemical structure of toluene. It is composed of a ring structure known as a benzene ring. Conventionally the benzene ring has the molecular formula C6H6. However, in the case of toluene, one of the hydrogens is substituted with a methyl group (--CH3). This mono-substitution of benzene with a methyl group converts it into a molecule with the molecular formula C6H5CH3.
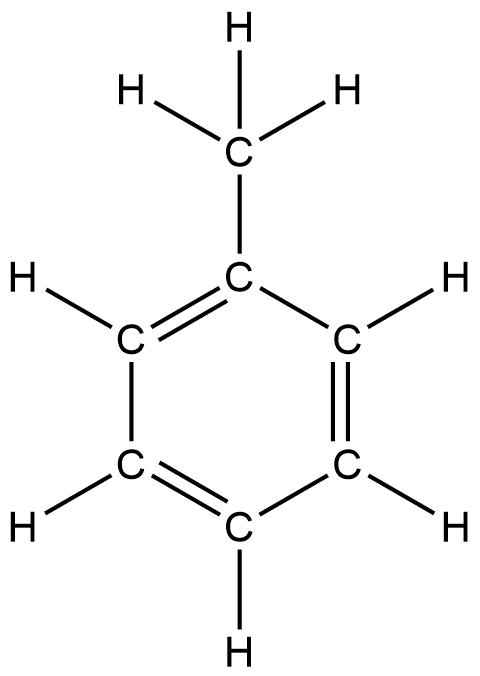
This is a more open structure of toluene for easier understanding of the difference between sigma and pi bonds.
All the hydrogens here are connected via a sigma bond to their respective carbons. If we count the number of hydrogens in this structure we notice 8 sigma bonds here.
Additionally, each carbon is connected to every other carbon by at least one bond. The first bond that each carbon forms with the other is also a sigma bond. Now since there are 7 carbons, it adds up to 7 more sigma bonds. This brings the total to 15 sigma bonds.
Now we can see that there are 3 bonds formed as superpositions on the 3 sigma bonds. They represent pi bonds.
Conclusion
Toluene has both sigma and pi bonds. The electrons of the pi bond participate in resonance thereby enhancing the stability of the whole system. Toluene has 15 sigma bonds of which 8 are between carbon and hydrogen and 7 of them between carbon-carbon. Toluene also has 3 pi bonds. The pi bonds are present in an alternate manner creating a conjugated system.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters