How Many Salts are There in Chemistry
Introduction
In this article, we will discuss what salt is, its types, and how many they are. Most of the salts are used in our daily life activities, without them we can’t do most of our work. For example, the most commonly used salt is Sodium Chloride(NaCl) which is commonly known as table salt. Likewise, there are so many types of salts. Before going to the types of salts let's find out the salt definition.
What is Salt?
Salt is a substance which is formed by the reaction of acid and base. The reaction is known as neutralisation. In this reaction, the cation from acid and anion from base substances react with each other to form water and salt.
\begin{equation}
\mathrm{NaOH}+\mathrm{HCl} \rightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}
\end{equation}
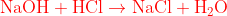
Types of Salts
The salts are classified into five types. They are
Acidic salts
Basic salts
Neutral salts
Mixed salts
Double salts
Acidic Salts
The acidic salts are formed by the reaction between strong acid and weak base reactants. In this reaction, the acid reactant remains incomplete in neutralisation due to the weak base.
In these, the hydrogen ions are excess, so it is Acidic.
\begin{equation}
\mathrm{NH}_3+\mathrm{HCl} \rightarrow \mathrm{NH}_4 \mathrm{Cl}
\end{equation}
![]()
Basic Salts
The basic salts are formed by the reaction between a strong base and weak acid reactants.
In this reaction, the base reactant remains incomplete in neutralisation due to the weak acid, and in these, the hydroxyl ions (OH–) ions are present in more than one or at least one. These basic salts are also known as Alkali salts.
\mathrm{NaOH + CH_3COOH \rightarrow CH_3COONa + H_2O}
![]()
Neutral Salts
The Neutral salts are formed by the reaction between the strong acid and strong base (or) the weak acid and weak base reactants. The hydrogen (H+ ) ions and hydroxyl (OH- )ions are equal in this. The pH of neutral salts is around 7.
\begin{equation}
\mathrm{NaNO}_3+\mathrm{HNO}_3 \rightarrow \mathrm{NaNO}_3+\mathrm{H}_2 \mathrm{O}
\end{equation}
![]()
Mixed Salts
The mixed salts are formed by the reaction between two cations sharing commonly with one anion or two anions sharing commonly with one cation reactants. In this, the reactants share commonly with their opposite ion to form mixed salts.
\begin{equation}
\mathrm{CaCl}_2+\mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CaOCl}_2+2 \mathrm{HCl}
\end{equation}
![]()
Double Salts
The double salts are formed by the reaction between more than one cation or anion. These salts are in a solid state which is more stable than others.
\begin{equation}
\left.\mathrm{K}_2 \mathrm{SO}_4\right)+\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3 \rightarrow \mathrm{K}_2 \mathrm{SO}_4 \cdot \mathrm{Al}_2\left(\mathrm{SO}_4\right)_3 \cdot 24 \mathrm{H}_2 \mathrm{O}
\end{equation}
![]()
Important Points
The salts are classified into types by the following properties:
Colour - most of the salts are crystal clear which means they are transparent, some salts are in colours because different anions and cations have different colours.
Odour - strong salts don’t have any odour whereas, the weak salts contain odour for acetates like vinegar and for cyanides like almonds and ammonium salts like ammonia.
Taste - the taste of the salts is different to each other, generally there are five tastes of the salts. Like Salty, sweet, Bitter, Sour, and Savoury.
Conductivity - generally the salts are conductors. The solutions of salts may produce electrolytes.
Melting point - most of the salts have the highest melting point. Different salts have different melting points. For example, the common salt, NaCl melts at 801°C. But, some salts can also melt at more or less room temperature.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
