How Many Primary Amines Are Possible For The Formula C_{4}H_{11}N ?
Introduction to Amines
Amines can be derived when there is the replacement of one or more hydrogen atoms of an ammonia molecule with an alkyl group and amines are also known by a functional group with a nitrogen atom having a lone pair which is also denoted by LP and it also resembles ammonia structurally where nitrogen can also bond up to 3 hydrogen atoms. Amines are also characterized by various properties that are based on carbon ( C) connectivity.
The compounds of nitrogen which are connected to a carbonyl group are called as amides and they have a structure R–CO–NR′R″ and may vary in different properties with amines.
Amines comes under organic compounds which contain nitrogen atoms and a lone pair and they are derived from ammonia \lgroup\:NH_3\rgroup ![]() in which one or more than one hydrogen atom is always replaced by an alkyl or any aryl group, so they are known by alkylamines and arylamines respectively.
in which one or more than one hydrogen atom is always replaced by an alkyl or any aryl group, so they are known by alkylamines and arylamines respectively.
Amine Structure
Nitrogen has five valence electrons so it is known as trivalent having a lone pair. As per VSEPR theory, the nitrogen atom is present in amines as sp^{3} ![]() hybridized and because of the presence of the lone pair, it is pyramidal instead of tetrahedral shape, a general structure for most of the sp^{3}
hybridized and because of the presence of the lone pair, it is pyramidal instead of tetrahedral shape, a general structure for most of the sp^{3} ![]() hybridized molecules. Each of the three sp^{3}
hybridized molecules. Each of the three sp^{3} ![]() hybridized orbitals of nitrogen atom is overlap with orbitals of hydrogen (H) or carbon (C) depending upon the configuration of amines and due to the presence of the lone pair, the C-N-H angle in amines is always less than 109 degrees because of the characteristic angle of tetrahedral geometry .
hybridized orbitals of nitrogen atom is overlap with orbitals of hydrogen (H) or carbon (C) depending upon the configuration of amines and due to the presence of the lone pair, the C-N-H angle in amines is always less than 109 degrees because of the characteristic angle of tetrahedral geometry .
Occurrence of Amines
Naturally, amines occur in the form of proteins, vitamins, hormones and they are also prepared synthetically for making the polymers, drugs, and dyes.
Types of Amines
On the basis of how the hydrogen atoms are replaced by an ammonia molecule the amines can be classified into 4 types they are-
Primary Amines
When one of the hydrogen atoms of the ammonia molecule is replaced by an alkyl or aryl group it is called primary amines.
Examples are Methylamine CH_3NH_2 ![]() , Aniline C_6H_5NH_2
, Aniline C_6H_5NH_2 ![]()
Secondary Amines
Two organic substituents replace the hydrogen atoms of the ammonia molecule forming an amine group it is called as secondary amines.
Examples are Dimethylamine \lgroup\:CH_3\rgroup_2NH ![]() , Diphenylamine \lgroup\:C_6H_5\rgroup_2NH
, Diphenylamine \lgroup\:C_6H_5\rgroup_2NH ![]()
Tertiary Amines
When all the three of the hydrogen atoms are replaced by an organic substituent, it could be an aryl or aromatic group called tertiary amines.
Examples are Trimethylamine N\lgroup\:CH_3\rgroup_3 ![]() , Ethylenediaminetetraacetic acid (EDTA)
, Ethylenediaminetetraacetic acid (EDTA)
Cyclic Amines
These are secondary or tertiary amines in an aromatic ring structure called cyclic amines.
Examples are Piperidine \lgroup\:CH_2\rgroup_5NH ![]() Aziridines C_2H_5N
Aziridines C_2H_5N ![]()
Basicity of Amines
Similar to ammonia, primary & secondary amines have protic hydrogens and thus they showcase a degree of acidity and on the other hand tertiary amines have no protic hydrogen and they do not possess a degree of acidity.
pK_{a} ![]() The value for primary & secondary amines is about 38, which makes amines a real weak acid. On the other hand if we take the pK_{b}
The value for primary & secondary amines is about 38, which makes amines a real weak acid. On the other hand if we take the pK_{b} ![]() It is about 4 and this makes the amines much more basic than acidic and the aqueous solution of an amine is strongly alkaline.
It is about 4 and this makes the amines much more basic than acidic and the aqueous solution of an amine is strongly alkaline.
Uses of Amines
Amines have many applications in our day to day lives. Some of the uses of amines are given below:
Amines are mostly used in the purification of water, even for the manufacturing medicine and development of insecticides and pesticides.
Amines are also involved in the production of amino acids which are responsible for the building block of proteins in living beings and amines also make many varieties of vitamins .
Serotonin is an important amine that mostly functions as one of the primary neurotransmitters and it basically controls the feelings of hungriness and it is also critical for the speed in which the brain operates in general.
Morphine and Demerol are considered under pain-relieving medicines which are also called as analgesics which are made from amines.
Main Content
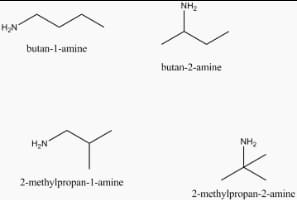
Four primary amines are possible with the formula C_{4}H_{11}N ![]() they are given below-
they are given below-
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
