How Many Neutrons Does Helium Have
Introduction
Neutrons are fairly massive patches, one of the nexus's primary ingredients. At the same time, electrons have nearly no mass. Both protons and mineral protons are positive. The neutron is one of the two constituent corridors of an infinitesimal nexus. Each matter comprises titles, containing three prominent patches of neutrons. Chargers' neutrons have a mass of 1.
Explanation
Neutrons
Helium
Neutrons
Neutrons that describe the relations of nucleons that are far piecemeal break down when the patches pair up and interact at close range. The terrain within the nexus of a single snippet seems analogous, with protons and neutrons also dancing about. But because the nexus is such a compact space, scientists have plodded to jut down the geste of these patches, known as nucleons, in a snippet’s nexus. A neutron is a subatomic flyspeck set up in the nexus of every snippet except that of simple hydrogen. The flyspeck derives its name from the fact that it has no electrical charge; it's neutral. Neutrons are highly thick.
Helium
It's a tintless and odourless inert gas that has unique parcels. Helium is a cooling medium for the Large Hadron Collider( LHC) and the superconducting attractions in MRI scanners and NMR spectrometers. Helium is the alternate most abundant element in the macrocosm after hydrogen. It's also used to keep satellite instruments cool. It was used to cool the liquid oxygen and hydrogen that powered the Apollo space vehicles.
Helium is also used to descry leaks, similar to auto air-exertion systems, and because it diffuses snappily, it's used to inflate auto airbags after impact. Hydrogen was formerly used to fill balloons, but it's dangerously reactive. Because it's veritably unreactive, helium gives an inert defensive atmosphere for making fibre optics and semiconductors and for bow welding. Because of its low viscosity, helium is frequently used to fill ornamental balloons, rainfall balloons and airships.
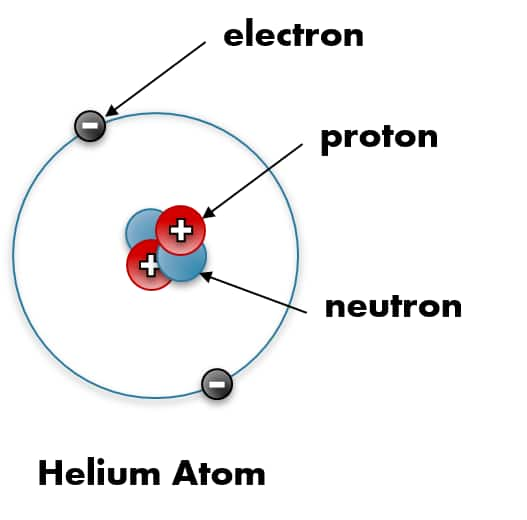
![]()
Helium atom proton electric charge diagram.
Conclusion
Atom:
An atom's atomic number (Z)
 is the number of protons present in that atom.
is the number of protons present in that atom.The number of protons and neutrons will be the same for a neutral atom.
An atom's mass number (A)
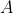 is the sum of protons and neutrons present in that atom.
is the sum of protons and neutrons present in that atom.
Helium element has atomic number 2 with the symbol He ![]() which belongs to the noble gas group, with 2 protons and 2 electrons.
which belongs to the noble gas group, with 2 protons and 2 electrons.
Helium:
The given number of protons is two are the atomic number of the He
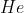 atom is 2
atom is 2The given mass number is 4u
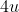 , which means the sum of protons and neutrons present in that atom.
, which means the sum of protons and neutrons present in that atom.So, the number of neutrons is the difference between the mass number and the number of protons.
The number of neutrons is 4 – 2 = 2 ![]()
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
