How Many Lone Pairs Does Oxygen Have
Introduction
Oxygen has 2 Lone Pairs. Lone pairs and bond pairs are the two different types of electron pairs. Lone pairs are electron pairs that do not form any bonds or are not involved in bonding; bond pairs are electron pairs that do form bonds or are interested in bonding between atoms. An oxygen atom has three valence electron pairs since it has six valence shell electrons. Since oxygen creates two bonds, we know that two electrons are required to develop those two bonds. There are now only two electron pairs left that are not involved in bonding.
Explanation
In general, you can use two methods to count the lone pairs. Learning the common bonding patterns of the elements in the second row and recognising the number of lone pairs and formal charges based on them is the first one, which is also what you should finally aim for. These patterns for the frequent bonding and standard charge combinations are listed in the following table:
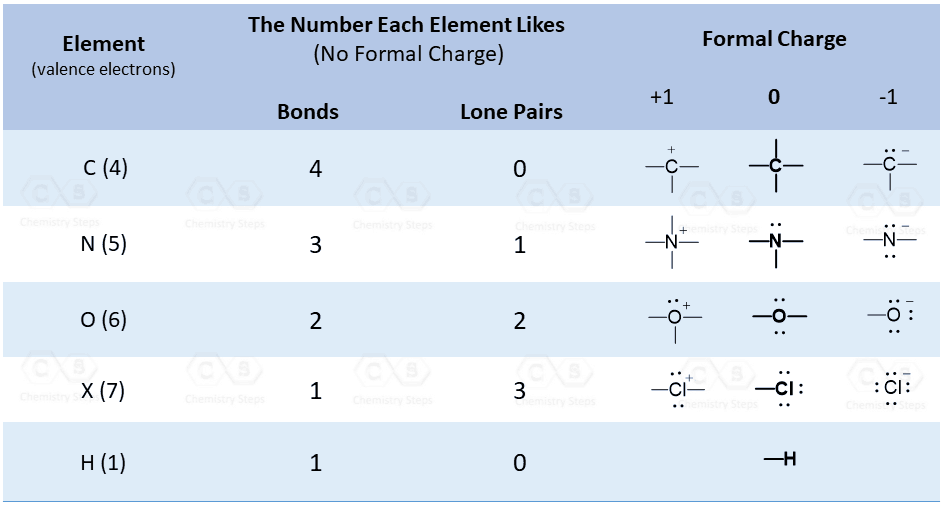
The second method is solving an equation with one unknown using the standard charge formula to find the number of lone pairs. From the last post, we learned that the formal charge might be determined using the following formula:
FC=V−(N+B)
Where :
V – number of valence electrons
N – number of nonbonding electrons
B – number of bonds
Conclusion
We are aware that an atom or molecule contains two different forms of electron pairs, namely lone pairs and bond pairs.
The electron pairs, known as "lone pairs", do not contribute to forming chemical bonds (covalent bonds) between two atoms. They are also referred to as non-bonding electrons or unshared pairs of electrons. They are always found in the atom's outermost shell.
However, the bond pairs are the pairs of electrons used to form chemical interactions, such as covalent or coordinate bonds, between atoms or molecules.
The oxygen atom's electrical structure is as follows:
1s2,2s2, 2p4
The second shell of the oxygen atom, which has 2s and 2p orbitals, is its outermost shell. An oxygen atom has three valence electron pairs since it has six valence shell electrons.
Since oxygen creates two bonds, we know that two electrons are required to develop those two bonds. There are now only two electron pairs left that are not involved in bonding.
Oxygen, therefore, has two lone pairs.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
