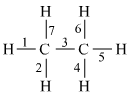How Many Covalent Bonds are Present in Ethane
Introduction
Chemistry is a branch of science that deals with the study of matter and the components that make it up. It also covers the properties of existing substances and the reactions that result in the creation of new elements. Chemistry's primary topics are atoms, ions, and molecules, which in turn make up elements and compounds. These chemical species typically converse with one another through chemical bonds. It is crucial to keep in mind that the interaction between matter and energy is a topic covered in chemistry.
Chemistry is the study of how elements and compounds can change, including the energy released or absorbed during those changes, as well as their properties, compositions, and structures.
The five primary subfields of chemistry are physical chemistry, organic chemistry, inorganic chemistry, analytical chemistry, and biochemistry.
Chemical Bonding
When two or more atoms, molecules, or ions join together chemically to form a compound, this process is known as chemical bonding. These chemical bonds hold the atoms in the resulting molecule together.
Chemical bonding is the pulling power that binds various parts (atoms, ions, etc.) together and stabilises them through complete energy loss. Chemical compounds depend on the strength of the bonds that connect their components; the more stable the final molecule, the stronger the bonds that bind the parts together.
The opposite is also true; if the chemical bonds between the constituents are weak, the resulting product will lack stability and will easily undergo another reaction to generate a more stable chemical complex (containing stronger bonds).
Types of Chemical Bonds
Ionic Bonds
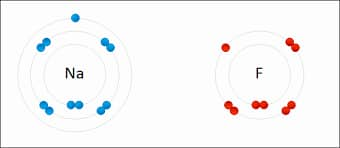
Ionic chemical bonds develop when electrons are moved from one atom or molecule to another. In this case, an atom loses an electron, which is subsequently gained by another atom. As a result of such an electron transfer, one of the atoms gains a negative charge and is dubbed an anion. A positive charge is added to the second atom, which is called the cation. The strength of the ionic bond increases with the size of the charge difference between the cation and the anion. The difference in charge between the two atoms strengthens the ionic interaction.
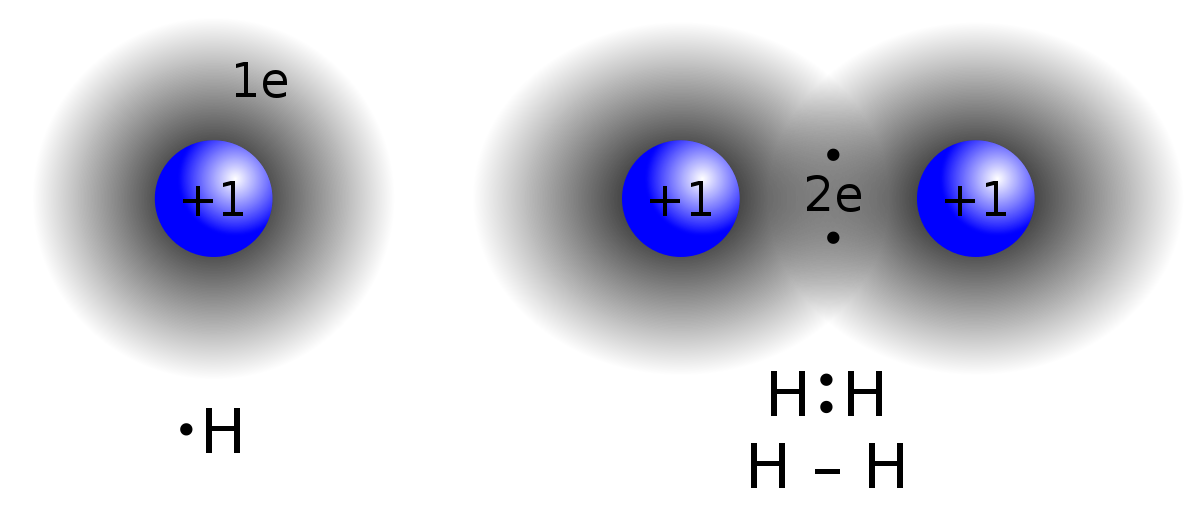
Covalent Bonds
Covalent connections are described as the sharing of electrons between atoms. Molecules containing carbon, which are typically referred to as organic compounds, frequently exhibit this type of chemical bonding. The shared pair of electrons between the two atoms have now wrapped around their nucleus, creating a molecule.
Hydrogen bonds
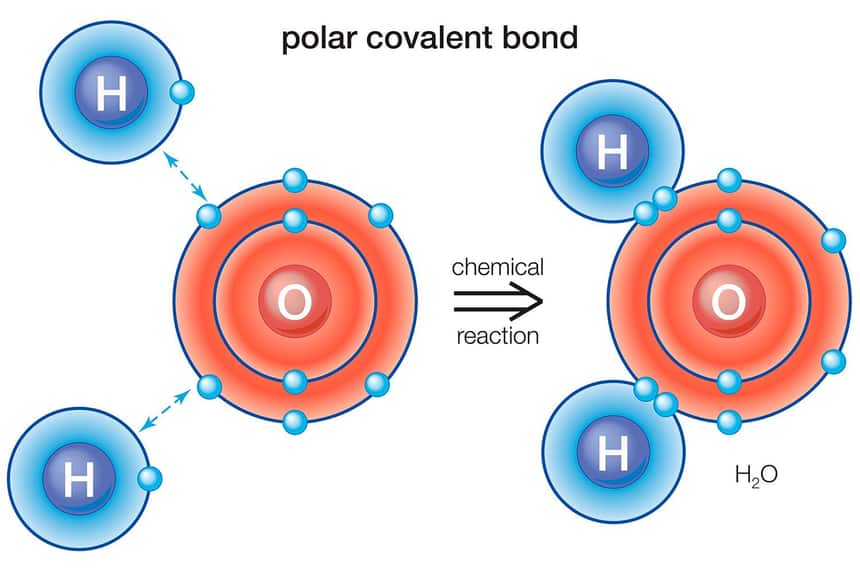
Ionic and covalent bonds are stronger types of chemical bonding than hydrogen bonds. Hydrogen acquires a little positive charge when it forms a polar covalent bond with oxygen. This implies that the electrons are bringing the more electronegative oxygen atom closer.
As a result, hydrogen has the propensity to be pulled to any adjacent atoms with negative charges. Many of the properties of water are due to a type of chemical bonding called the hydrogen bond.
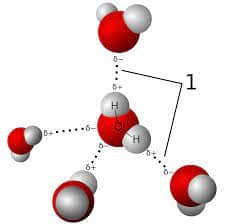
Polar Bonds
Covalent bonds can be either polar or non-polar. Because the more electronegative atom pulls the electron pair away from the less electronegative atom and toward itself, there is an unequal distribution of electrons in polar covalent chemical bonds. Water is called a polar molecule.
There is a discrepancy in charge in different sections of the atom due to the unequal distribution of electrons among the atoms. The ends of the molecules are often partially charged on one end and partially charged on the other.
Hydrocarbons
An organic substance known as a hydrocarbon is formed completely of the two atom types of carbon and hydrogen. Typically, hydrocarbons are colourless gases with hardly audible odours. The four subcategories generally in hydrocarbons are alkanes, alkenes, alkynes, and aromatic hydrocarbons. The structures of these substances could be quite straightforward or very complex. Saturated, unsaturated, cycloalkanes, aromatic, and aliphatic hydrocarbons are some of the several forms of hydrocarbons.
Saturated Hydrocarbons
Carbon-carbon atoms and carbon-hydrogen atoms are joined by single bonds in these molecules. The simplest hydrocarbons are those with a single bond. There are no double or triple bonds in these kinds of hydrocarbons.
Ethane
Ethane is a saturated hydrocarbon that occurs as a gas. The most straightforward alkane is methane, with ethane following in second. There are 6 hydrogen atoms and 2 carbon atoms in it. It is created in a lab setting using sodium propionate. Ethane is the most essential gaseous fuel. It is simple to remove ethane and other heavier hydrocarbons from the gas stream and liquefy them at moderate pressure. There are covalent bonds in ethane.
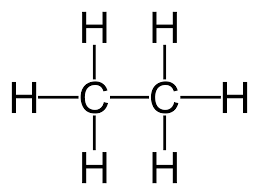
Covalent Bonds Present In Ethane
Here from the above chemical diagram since there are six covalent bonds present between carbon and hydrogen atoms and one covalent bond present between carbon atoms. Therefore the total number of covalent bonds present in ethane is seven.
Conclusion
Chemistry studies how elements and compounds can change, including the energy, released or absorbed during those changes, as well as their properties, compositions, and structures. Atoms are known as the "basic building blocks of matter." Molecules are made up of one or more atoms connected by covalent (chemical) connections. When two or more atoms, molecules, or ions join together chemically to form a compound, this process is known as chemical bonding. Ethane is a saturated hydrocarbon that occurs as a gas.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters