Ninhydrin Test - Definition, Reaction, Principle, Result, FAQs
Definition of the Ninhydrin Test
The ninhydrin test is a chemical test used to determine the presence of ammonia, primary and secondary amines, or amino acids. In this test, ninhydrin reagent is added to the test sample, resulting in the production of a deep blue colour, also known as Ruhemann's purple, in the presence of an amino group.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Definition of the Ninhydrin Test
- What exactly is the Ninhydrin Test?
- Ninhydrin Test Results and Interpretation
Ninhydrin reagent composition
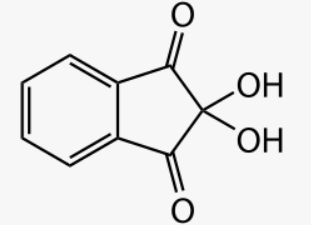
Ninhydrin reagent is known as 2,2-dihydroxyindane-1,3 dione.
Ninhydrin structure
Define deamination
Deamination is defined as the removal of alpha amino group from the molecule.
What exactly is the Ninhydrin Test?
The ninhydrin test is a chemical test that determines if an analyte contains amines or -amino acids chemical test solution is combined with ninhydrin in this assay (a chemical compound with the formula C9H6O4 and the IUPAC name 2,2-dihydroxybenzene-1,3-dione). The presence of ammonia, primary and secondary amines, or amino acids in the analyte is indicated by the development of a deep blue colour.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Imino acid example
The molecule containing both imine group and a carboxylic acid group present in the same carbon atom is referred as imino acid group. Example: proline
Ninhydrin Test Objectives
The presence of amines and amino groups in the test solution must be detected.
To determine the number of amino acids in a sample.
To differentiate between carbs and amino acids.
Ninhydrin and its Analogy
Ninhydrin is the most frequent chemical reagent used to detect latent fingermarks on porous materials such as paper and cardboard. The molecule combines with the amino acid (eccrine) component of the fingerprints deposit to produce Ruhemann's purple, a dark purple result. The chemical processes involved are highly complex, and development parameters such as temperature, acidity (pH), and humidity must be regulated in order to achieve the best outcomes.
Principle of the Ninhydrin Test
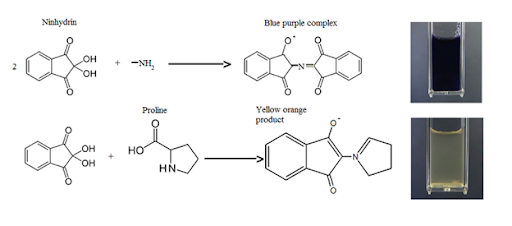
The amino acid oxidative deamination when exposed to ninhydrin, releasing CO2, NH3, and an aldehyde in order to hydrindantin (which is a reduced form of ninhydrin). The ammonia then reacts with another ninhydrin molecule to form diketo hydrin (also known as the Ruhemann's complex). The ammonia then reacts with another ninhydrin molecule to form diketo hydrin (also referred to as the Ruhemann's complex). The intense blue colour is due to this complex. When Amino-acids such as proline are present in the analyte, a yellow complex is generated. When asparagine is employed, the resultant complex is brown in colour.
Ninhydrin reaction is shown below.
Ninhydrin reaction with amino acids
Requirements and reagents
Ninhydrin reagent Ninhydrin reagent: Dilute 0.35g of ninhydrin in 100 mL of ethanol (isopropanol or a 1:1 mixture of acetone and butanol can be used in place of ethanol).
Solvent for dilution (for quantitative testing): Combine equal parts water and n-propanol.
Typical solution (1 percent protein solution)
Materials needed
Tubes for testing
Stand for test tubes
Pipette
Equipment
A bath in water
Spectrophotometer
Ninhydrin Test Methodology
For qualitative research
In one dry test tube, place 1 ml of standard protein solution and 1 ml of test sample.
In both test tubes, add a few drops of ninhydrin reagent.
Place the test tubes in the water bath for 5 minutes before allowing them to cool to room temperature.
Observe the creation of colour and make a note of the outcome.
For quantitative research
Pipette varying volumes (10 l, 20 l, etc.) of the protein solution from the supplied stock solution into a series of test tubes then distil the volume to 1 mL.
Take a blank tube with 1ml of distilled water and the rest of the tubes labelled 2 to 9 for the building of a standard curve. Tubes 10-15 are for unidentified samples.
In each tube, add 1 mL of the ninhydrin reagent and 5 mL of the diluent solvent and thoroughly mix by vertexing.
Allow the tubes to cool.
Cover the tubes with covers and incubate for 17 minutes at 90°C or 20 minutes in a boiling water bath.
When measuring the optical density of the solutions against a blank at 570 nm, cool the tubes to ambient temperature (440 nm for proline and hydroxyproline).
Draw a standard curve of A570 on the Y-axis as well as the concentration of amino acid on the X-axis to quantify the amount of amino acid in the unknown compound.
| Related topics link, |
The next five steps explain how alpha amino acids combine with Ninhydrin, which is involved in the formation of colour.
1. alpha-amino acid + ninhydrin ninhydrin reduced +Alpha amino acid +H2O
This is an oxidative deamination reaction that produces an alpha – amino acid by eliciting two hydrogens from the alpha amino acid. With the creation of a water molecule, the ninhydrin is also reduced and loses an oxygen atom.
2. alpha-amino acid + H2Oalpha-keto acid + NH3
Rapid hydrolysis of the NH group inside the alpha-amino acid produces an alpha-keto acid containing an ammonia molecule. This alpha-keto acid was also engaged in the step's decarboxylation reaction.
3. NH3 + alpha-keto acid aldehyde + CO2
The overall reaction for the preceding reactions is easily summarised in Reaction (4):
4. alpha-amino acid + 2 ninhydrins CO2 + aldehyde + final complex (BLUE) + 3H2O
In summary, ninhydrin, which is initially yellow, combines with amino acids and changes to a deep purple colour. This purple colour is recognised by this method.
Under heated conditions, to create an aldehyde with one fewer carbon atom than the original amino acid. Along with the aldehyde, a carbon dioxide molecule is formed. These first three processes result in lower ninhydrin and ammonia, which are necessary for colour creation.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
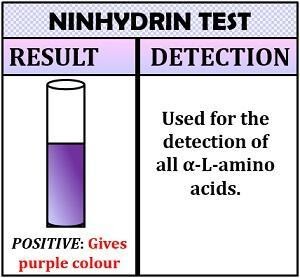
Ninhydrin Test Results and Interpretation
Test for Ninhydrin
The presence of a purple-coloured complex in the tube indicates a good result and the existence of amino acid in the sample.
The absence of the compound in the tube is a negative outcome, indicating that the sample lacks amino acids.
We can deduce the unknown concentration samples from the graph.
Ninhydrin Test Applications
The ninhydrin test detects the presence of amino acids in unknown substances.
This assay is also used in solid-phase peptide synthesis to check the protection of proteins for amino acid analysis.
The ninhydrin test is often used to detect fingerprints due to its high sensitivity. It is conceivable because ninhydrin reacts with the terminal amines of lysine residues in peptides and proteins shed off in fingerprints.
Ninhydrin Test limitations
Ninhydrin reacts with -amino groups as well as nitrogen in ammonia and other free amines.
Because steric hindrance prevents ninhydrin from reaching the -amino groups, the Ninhydrin test is ineffective for detecting high molecular weight proteins.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The purpose of a ninhydrin test is to confirm the presence of an amino group in a given molecule. We will need ninhydrin reagent, test tubes, a carrier solvent such as ethanol or distilled water, a spatula, a warm water bath, and the test sample to perform the ninhydrin reaction. The following are the steps to carry out the reaction:
Using a carrier solvent such as acetone or ethanol, we make a 2% solution of ninhydrin. We achieve this by dissolving 0.2g of ninhydrin in 10ml of the solvent.
Then, using distilled water, we make a solution of the indicated test chemical.
Then, using distilled water, we make a solution of the indicated test chemical.
We put the test solution in a test tube and add a few drips of the ninhydrin solution to it. We let the test tube settle for a few minutes in a water bath at a slightly increased temperature.
We have a positive ninhydrin test result if the colour of the solution shifts to a deep blue coloration.
The mechanism of the ninhydrin reaction is simply an oxidation and reduction reaction process. When we add droplets of ninhydrin solution to a test sample, the ninhydrin works as an oxidising agent. It interacts with the compound's amino group, causing delamination. This procedure produces two gaseous by-products: ammonia and carbon dioxide. In addition to producing an aldehyde, this redox reaction reduces the ninhydrin and produces a reduced product known as hydrindantin. The ammonia that has been released now interacts with another molecule of ninhydrin to generate a di-ketohydrin complex with a deep blue coloration.
blue
A vivid blue coloration in the solution indicates a positive ninhydrin test. This reaction suggests that amino acids, other amines, and ammonia are present in the test material.
It causes irritation to mammalian skin. Various harmful effects of ninhydrin have been described in laboratory animals; nevertheless, no long-term in vivo bioassay has been performed to assess its carcinogenic and co-carcinogenic potential in laboratory animals.
Ninhydrin reacts with the skin, causing a blue stain due to the presence of amino acids in the skin. It is capable of detecting ammonia and amines. Because of the presence of amine as a functional group, ninhydrin is also used in protein detection.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM

