Ronsenmund Reduction Mechanism - Explanation, Reaction with FAQs
Rosenmund Reaction Class 12
Rosenmund Reduction reaction Mechanism deals with the selective reduction of acyl chlorides into aldehydes. Karl Wilhelm Rosenmund was the first person who reported the path in which acyl chlorides are selectively reduced into aldehydes in the year1918. thus, the reaction was named after the name of the scientist Rosenmund. The Rosenmund reaction is, basically, a hydrogenation process.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Rosenmund Reaction Class 12
- Rosenmund Catalyst
- Catalytic Reduction Mechanism
- Explanation of Mechanism
- Acid chloride to Aldehyde
Here, a molecule of hydrogen reacts with the acyl chloride in the presence of active palladium on barium sulfate catalyst. The role that barium sulfate plays is to reduce the activity of the palladium due to its low surface area. As a result, over reduction is prevented. If there arises further need of reduction in palladium activity (in case of more reactive acyl chlorides), a catalytic poison should be added to deactivate the palladium catalyst completely.
Thioquinanthrene and thiourea are two common poisons which can be used to reduce palladium activity in the Rosenmund’s reaction. The need for deactivation of the catalyst arises due to the subsequent aldehyde, formed from the reduction of the acyl chloride, further be reduced to form a primary alcohol by the catalyst. The primary alcohol thus formed, will react with the residual acyl chloride to produce an ester. Thus, yield of the desired product will be decreased subsequently.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Rosenmund Catalyst
The Rosenmund catalyst (palladium on barium sulfate) is prepared by reducing palladium(II) chloride (PdCl2) solution in presence of a reducing agent, such as, formaldehyde in the presence of barium sulfate. A Rosenmund catalyst is, usually used to carry out selective reduction of acyl chlorides to their corresponding aldehydes. This is characteristically composed of palladium supported on BaSO4, and sometimes, poisoned by sodium acetate, N,N-dimethylaniline, thiourea, thiophene, dibenzothiophene, ethyldiisopropyl amine, or, most commonly, quinoline.
Catalytic Reduction Mechanism
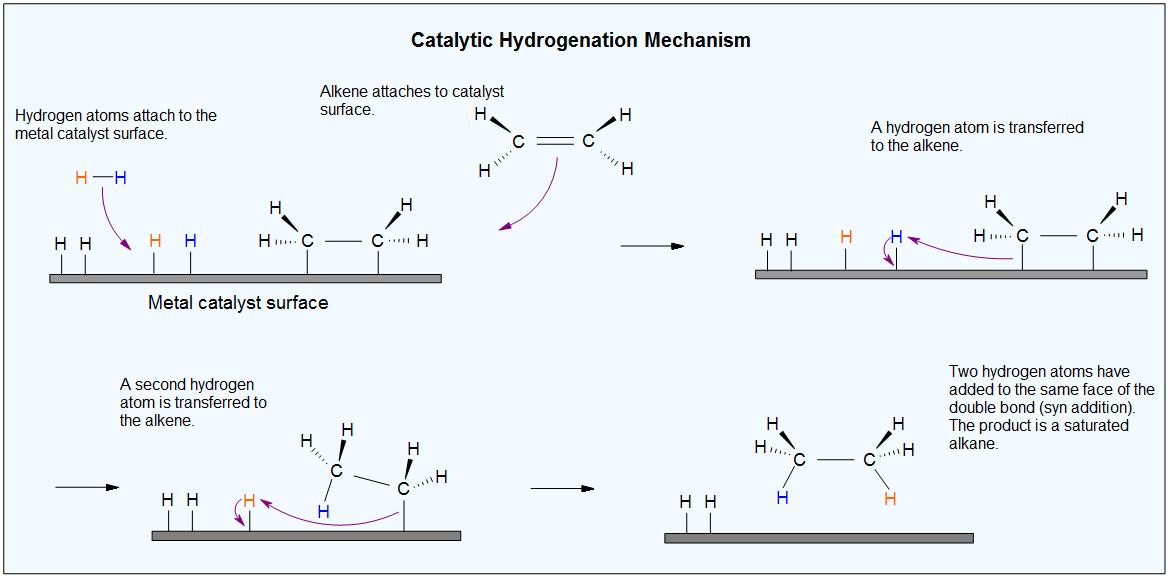
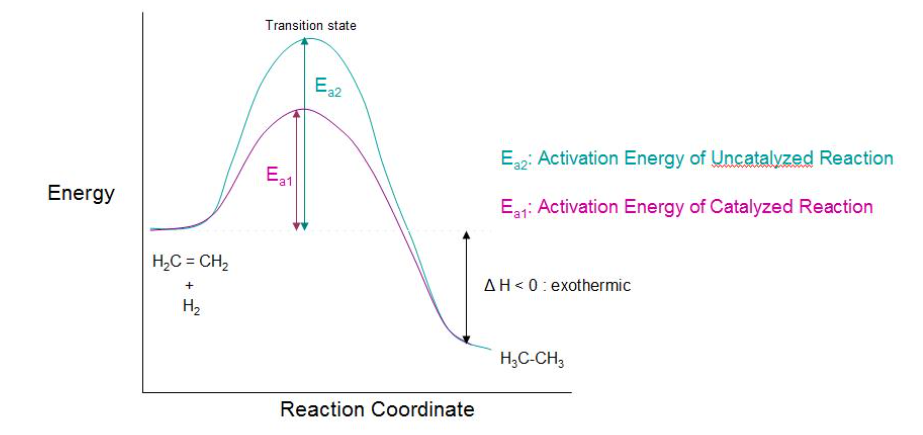
Addition of hydrogen to a double bonded carbon moiety to produce an alkane is a reduction reaction. This can happen in the presence of a catalytic amount of hydrogen, thus called catalytic hydrogenation. Catalytic Hydrogenation reaction is a thermodynamically stable reaction since, more stable (lower energy) product is obtained. Thus, it is an exothermic reaction (heat is released) as the product is more stable than reactant.
The heat thus released is termed as the heat of hydrogenation. The reaction between molecular hydrogen (H2) and an alkene (saturated hydrocarbon) requires an active metal catalyst. A catalyst enhances the rate of the reaction by lowering the activation energy of the transition state. Catalysts which are commonly used in catalytic reduction techniques are: platinum, palladium, and nickel.
The metal catalyst acts as a surface for adsorption of hydrogen on which the reaction takes place. In the presence of these catalyst, the sigma bond of H2 breaks, and the two hydrogen atoms instead bind to the metal. The π bond of the alkene also interacts with the active metal surface which insists the weakening of the π bond,
Now, both the reactants are bound to the metal surface. the hydrogen atoms can be easily added, one at a time, to the previously unsaturated (double bonded) carbons.
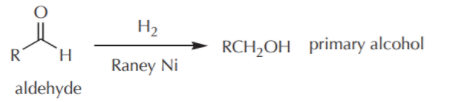
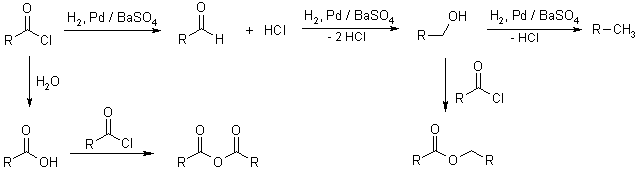
Catalytic hydrogenation of aldehydes and ketones
In the catalytic hydrogenation of Aldehydes, corresponding primary alcohols are produced.
here, Raney Ni is used as a catalyst.
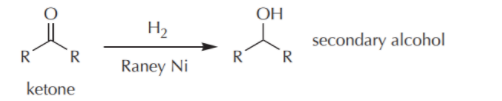
In the catalytic hydrogenation of ketones, a molecule of hydrogen is added across the carbon-oxygen double bond which leads to form a secondary alcohol as a final product.
here, Raney Ni is used as catalyst
Rosenmund reduction mechanism (Rosenmund reaction mechanism)
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Explanation of Mechanism
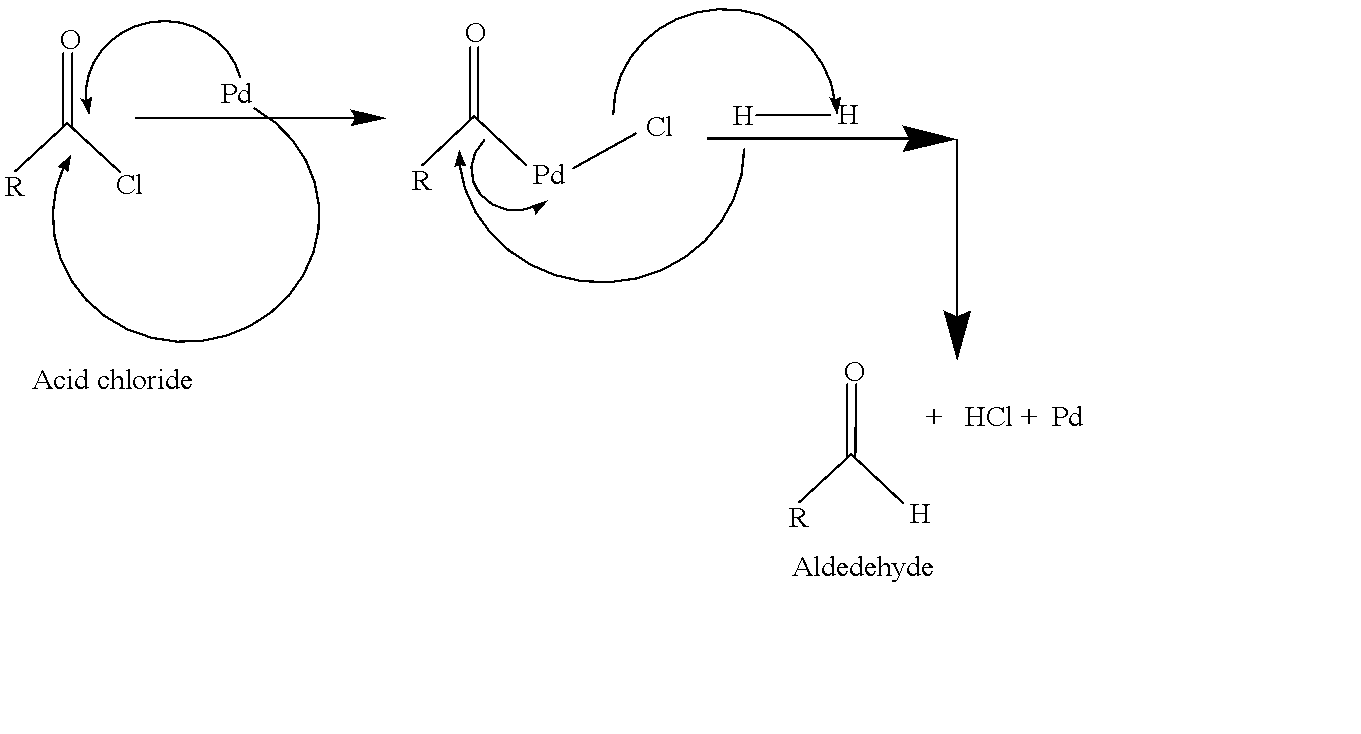
step 1: Hydrogenated Palladium (active metal) in presence of barium sulphate converts acid chlorides to acetaldehyde by selective reduction.
Initially, acetyl chloride binds with Pd surface and Pd binds in between C and Cl. the Pd(0) becomes Pd(II) after being bonded.
step 2: Cl binds with molecular hydrogen and gets eliminated by forming HCL. and another H atom binds with the carbonyl C which generates aldehyde as a major product.
step 3: Pd(0) is regenerated and further acts as a catalyst
step 4: in case of highly reactive acid chlorides, the over reduction by catalyst is prevented by using catalyst poisons, such as, thiourea, xylene etc.
step 5: barium sulphate reduces the activity of palladium metal by decreasing the surface are of adsorption.
| Related topics link, |
Examples:
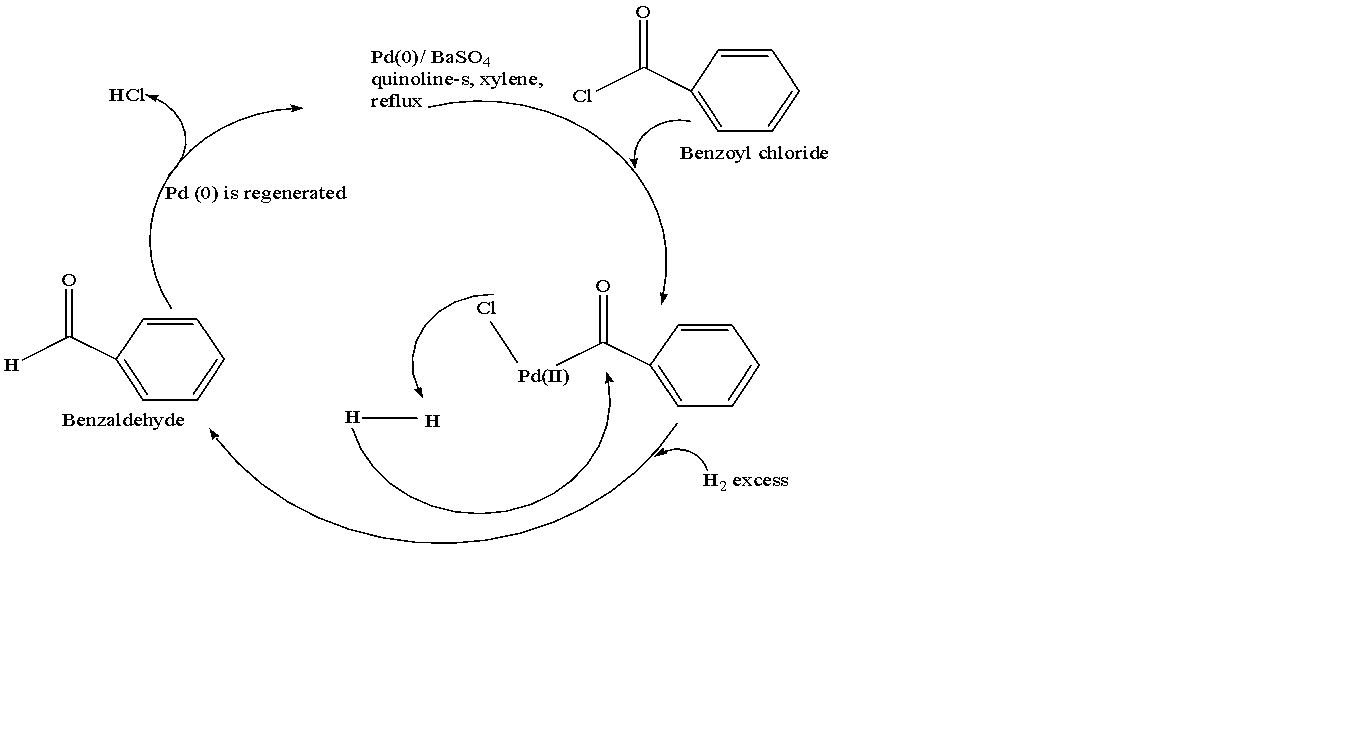
Hydrogenation of benzoyl chloride in presence of pd on BaSo4
Some catalytic poisons are added to the Rosenmund catalyst to stop further reduction once the desired product is obtained. An example for the method to completely deactivate the palladium over barium sulfate catalyst given below-
Acid chloride to Aldehyde
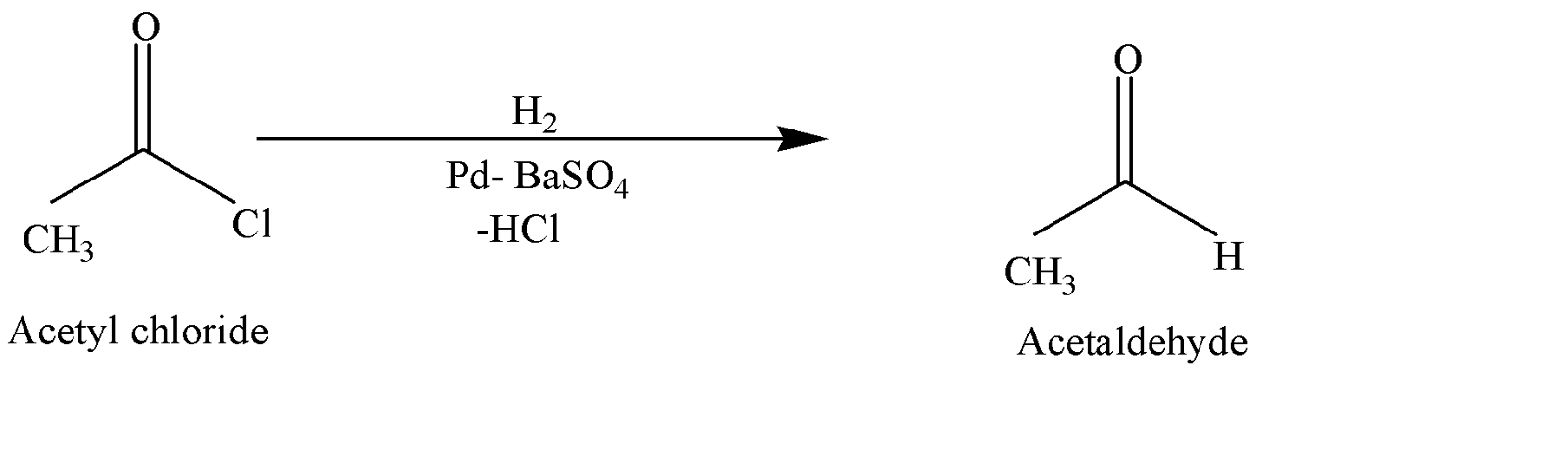
In the presence of active palladium metal in barium sulphate, acetyl chloride is reduced selectively to form acetaldehyde.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The conversion of acid chlorides into aldehydes in low temperature and low-pressure hydrogenation over poisoned Pd/BaSo4 is the classical Rosenmund reduction reaction.
The Rosenmund reduction reaction is catalysed by palladium on surface of barium sulphate. The barium sulphate decreases the activity of the palladium due to its low surface area, hence, prevents unwanted reduction.
Use of palladium, as a catalyst, persuades the reduction process. Whereas, use of barium sulphate decreases the activity of palladium. since, barium sulphate has low surface area, it prevents the over reduction of reactant and we get our desired product. So, To prevent further hydrogenation, palladium is mixed with poison.
The most abundant catalyst which is used in Rosenmund reaction is palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. Barium sulfate is used as catalyst poison.
Lindlar's catalyst is a palladium catalyst which is poisoned with pinch of lead and quinoline. It helps to reduce its activity such that it can only reduce alkynes, not alkenes.
Also Read
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:12 PM
02 Jul'25 05:10 PM

