Formaldehyde (CH2O) - Properties, Uses and FAQs
Introduction:-
Let us clear up the concept of aldehydes before learning about the formaldehyde formula.
Aldehydes are a class of organic compounds consisting of a carbon doubly bonded to oxygen i.e. carbonyl group at the centre. The carbonyl carbon is attached to hydrogen and an alkyl/aryl/hydrogen atom on either side. The aldehydes can be classified by the presence of the carbonyl group acting as the functional group itself. It is also typically known as the formal group. Click here to get more information about Aldehydes, Ketones and Carboxylic Acids.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs

Aldehydes are generally prepared by eliminating a hydrogen atom from alcohol and can be commonly identified as formaldehyde. Now, let us have a look at the formaldehyde formula.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
One of the simplest aldehydes is formaldehyde, also inferred as formalin or methanal. Formaldehyde structural formula is given as follows:-
Methanal or formalin is a flammable and colourless gas with a pungent smell. Methanal or formalin is a generally existing organic compound having a molecular mass of 30.026 g/mL. Formaldehyde structural formula or methanal formula is given as follows:-
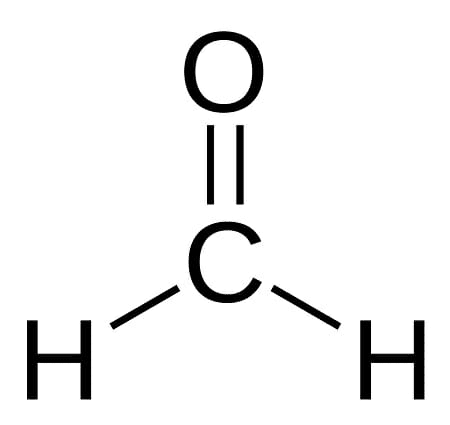
Formaldehyde or methanal gas further polymerises to form paraformaldehyde and so it is conserved as an aqueous solution. We all know that the formaldehyde chemical formula is CH2O. The bond between carbon and oxygen in the carbonyl functional group is polar, making the compound highly reactive. The oxygen atom of the carbonyl group is capable of attracting electrons more strongly than the carbon atom. This creates a partial negative charge and a partial positive charge at both the ends of the functional group. This causes it to stick to the polar particles and empowers it with some ability to attract and donate electrons.
Also Read:
Below are the NCERT Solutions class 12 chemistry, NCERT Exemplar Class 12 and NCERT notes for class 12 which can be assessed from the links given below.
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Some properties of formaldehyde are listed below:-
- Formation of salt formats and ethanol on reacting with a strong base like NaOH.
The chemical equation is represented below-
2HCHO + NaOH →HCOONa + CH3OH
Formaldehyde forms formidable and water in reacting with ammonia, and the reaction is given below-
6HCHO + 4NH3 → (CH2)6N4 + 6H2O
Now let us study the preparation techniques of methanal or formaldehyde:-
- Lab method:
A blend of methanol vapours and the air is passed over platinumised asbestos or silver or copper catalyst at 300°C.
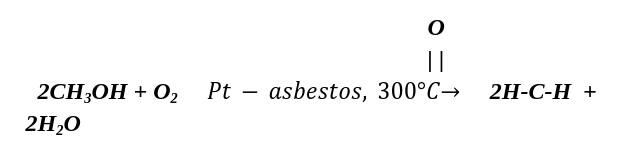
- Industrial method:
A blend of methanol vapours and the air is passed over iron oxide – molybdenum oxide at 500°C.

Methanal structural formula:-
Methanal structure and methanal formula is same as formaldehyde i.e. CH2O.
Methanal is nothing but a 37% (w/w) aqueous solution, furthermore known as formalin. It usually consists of methyl alcohol and an inhibitor which is usually an ethyl (vinyl) polymer to prevent it from further polymerization. The most important use of methanal is in the manufacture of methanal resins. Methanal is mostly produced by a process that involves both dehydrogenation and oxidation.
August von Hofmann discovered methanal in 1867. The majority of methanal exist as formalin and is used in the production of dyes and plastic material.
In the early days, chemical compounds were frequently named on the subject of how they have been derived, or maybe, where they have been derived. Even the factors maintain the historical terminology of their symbols, like Fe (Ferro) for iron and Hg (mercury). As this case illustrates, we've systematized the preceding useful organization designations (aldehydes are represented with the aid of using the suffix-“al” in preference to the complete word).
As an increasing number of new compounds (and factors) have been located or made, a consistent nomenclature system has become more important to transfer knowledge in the textual aspect. Hence, IUPAC (International Union of Pure and Applied Chemistry) was established to construct nomenclature rules.
So, the word formaldehyde was derived from formic acid.
| Related topics link, |
Formalin:-
Formalin chemical formula is CH2O; same as formaldehyde formula. Formalin is usually used as a reducing agent for producing formic acid as it has a neutral pH. It also has its application as herbicides and disinfectants. It is so used because it inactivates viruses and toxins when used in a vaccine. Further, it is used in cosmetic products.
This aqueous solution of formaldehyde or methanal is termed formalin. Formalin formula is the same as formaldehyde i.e CH2O.

Formalin is nothing but formaldehyde/methanal dissolved in water. A 37% aqueous solution of methanal is usually used as formalin for various purposes.
People often get confused with formaldehyde and formalin, but forget about a basic difference between these two. Formaldehyde is a colourless gas while formalin is an aqueous solution of formaldehyde.
Let us have a look at the role of formaldehyde in human beings:
Formaldehyde plays an important role in the cellular metabolism of humans and mammals. A human can die after ingesting 30 ml of 37% of formaldehyde solution. Formaldehyde irritates the eyes when exposed to concentrations above 0.1 ppm. Inhaling this much amount of formaldehyde results in headaches, burning sensation, breathing problems and is fatal for asthmatic patients.
Uses of formaldehyde:-
- It is applied withinside the manufacture of resins like urea-formaldehyde and plastics along with bakelite.
- It is employed in the production of dyes – indigo, pararosaniline, etc.
- Also applied as a decolourising agent in barrel dyeing.
- Used in the production of urinary antibacterial which is urotropine.
- Used to process the polio vaccine.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Formaldehyde exists as a gas and formalin is an aqueous solution containing formaldehyde.
Exposure to relatively greater concentrations of formaldehyde can cause cancer according to laboratory tests conducted on lab animals.
Some populations may experience health issues when exposed to a concentration greater than 0.1 parts per million. They are listed below-
Watery eyes.
Burning sensations in eyes, mouth, throat
Coughing
Wheezing
Nausea
Irritation to skin.
Formaldehyde encompasses various forms which are interconvertible. They are-
Molecular formaldehyde – It is a colourless gas having a pungent odour.
1,3,5 Trioxane – It is a trimer of formaldehyde having molecular formula (CH2O)3. It exists as a white solid dissolving without getting degraded in organic solvents.
Paraformaldehyde – Formaldehyde gets converted to paraformaldehyde on polymerization. Again it resists as a white solid which is mostly insoluble in all solvents. The formula for paraformaldehyde is HO(CH2O)nH.
Formalin is used as a preservative. It is specially used to preserve biological specimens in zoological and medical laboratories. Formalin solution helps the specimens to stay as it is for a longer duration delaying the decaying process.
Exposure to a certain amount of formaldehyde irritates the eyes and burning sensations in the mouth.
Also Read
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:12 PM
02 Jul'25 05:10 PM

