How Many Single Bonds are Present in Benzene
Introduction
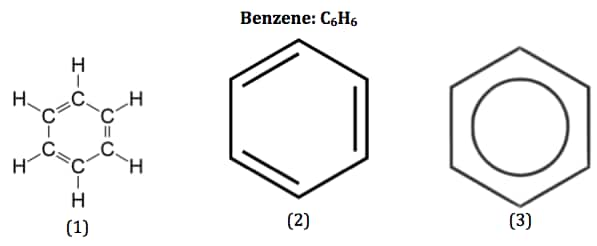
There are 9 single bonds present in benzene. The broad family of chemical molecules known as aromatic compounds, which includes benzene, has its origins in this substance. In contrast to cyclohexane, benzene only has six hydrogen atoms, which gives the perception that the ring is unsaturated and that each carbon atom is a part of one double bond. For benzene, two distinct configurations with alternating single and double bonds around the ring are possible.
Structure of Benzene
When benzene (C6H6) was initially found, researchers assumed it contained double or triple bonds due to its low hydrogen-to-carbon ratio (1:1). This reaction was used to add bromine (Br2) to benzene because double and triple bonds add bromine (Br2) quickly. Surprisingly, benzene has no reaction at all with bromine. In addition, benzene undergoes substitution reactions rather than the addition reactions typical of alkenes when it is driven to react with bromine through the presence of a catalyst. These tests revealed that the benzene core's six carbons are unusually resistant to chemical change.
For many years, it was unclear how to reconcile the conceptual paradox of high unsaturation (low H: C ratio) and high chemical stability for benzene and similar molecules. A hexagonal, planar ring of carbons with alternate single and double bonds was eventually chosen as the structure of benzene, and the outstanding chemical stability of this system was attributed to a unique resonance stabilisation of the conjugated cyclic triene. Since benzene is a mixture of two structurally and energetically equivalent resonance forms that depict the continuous cyclic conjugation of the double bonds, no single structure can accurately represent it. In the past, the pi-electron delocalisation of the benzene was symbolised by a circle in the centre of a hexagon to indicate the benzene resonance hybrid. Due to the fact that this approach does not reveal the pi electrons present in benzene, it has mostly been abandoned. Currently, one resonance form is typically used to depict the structure of benzene, with the understanding that this representation is not entirely accurate.
In benzene, the six-membered ring is a perfect hexagon with all carbon-carbon bonds measuring 139 pm in length. The C=C double bond length (139 pm) and the C-C single bond length (154 pm) are essentially equivalent, which is consistent with the benzene ring being a resonance hybrid made up of 1.5 C-C bonds. All of the C-C-C and H-C-C bond angles in benzene are 120 degrees because each carbon in the ring has undergone sp2 hybridization, which makes the entire molecule planar.
The High Stability of Benzene
Measurements of the heat generated when double bonds in a six-carbon ring are hydrogenated (hydrogen is added catalytically to produce cyclohexane as a typical result) have provided evidence for the greater thermodynamic stability of benzene. Cyclohexane is shown as a low-energy reference point in the graphic below. Cyclohexane is created when hydrogen is added to cyclohexene, and 28.6 kcal of heat is released in the process. We would anticipate that a cyclohexadiene would release 57.2 kcal per mole upon complete hydrogenation and a 1,3,5-cyclohexatriene would release 85.8 kcal per mole if we take this number to represent the energy cost of inserting one double bond into a six-carbon ring. These hydrogenation temps would represent the compounds' relative thermodynamic stability. 1,3-Cyclohexadiene is marginally more stable in practice than anticipated, by around 2 kcal, most likely as a result of the double bonds becoming conjugated. But benzene is remarkably 36 kcal/mole more stable than anticipated. Aromaticity is a type of stability improvement, and molecules having aromaticity are referred to as aromatic compounds. The most typical aromatic chemical is benzene, although there are numerous others as well. When compared to conventional alkenes, benzene is less reactive, which is due to aromatic stabilisation.
Atomic Orbitals of Benzene
Additionally, each of the six carbon atoms in benzene is sp2 hybridised and has an unhybridized p orbital that is perpendicular to the ring's plane. It is impossible for the six carbon atoms to only overlap with one neighbouring p orbital to form three specified double bonds since each of the six carbon atoms and its corresponding p orbitals is equivalent. Instead, there is a cyclic overlap involving all six p orbitals since each p orbital overlaps equally with both adjacent orbitals. This enables more overlap than would be possible with the linear 1,3,5-hexatriene counterpart by delocalizing the p orbitals into molecular orbitals that wrap around the ring. Naturally, for this to occur, the ring needs to be planar; otherwise, the p orbitals couldn't correctly overlap, yet it is well known that benzene is a flat molecule. The ring's pi electrons are evenly distributed throughout, as can be seen in the electrostatic potential map of benzene below, along with each carbon equivalent.
The Molecular Orbitals of Benzene
A more comprehensive and all-encompassing explanation of "aromaticity" can be found in the molecular orbital description of benzene. We are aware that benzene has a planar hexagonal structure with sp2 hybridised carbon atoms and equal-length carbon-carbon bonds. Following the overlap of the remaining six p-orbitals (one on each carbon) in the cyclic array, six molecular orbitals—three bonding and three antibonding—are created.
The plus and minus symbols refer to phase indications in the equations that define these orbitals rather than electrostatic charge (in the diagram the phases are also colour coded). When the phases line up, the orbitals overlap to create a region of like phase, with the most overlapping orbitals generating the zone.A more comprehensive and all-encompassing explanation of "aromaticity" can be found in the molecular orbital description of benzene. We are aware that benzene has a planar hexagonal structure with sp2 hybridised carbon atoms and equal-length carbon-carbon bonds. Following the overlap of the remaining six p-orbitals (one on each carbon) in the cyclic array, six molecular orbitals—three bonding and three antibonding—are created. The diagram's plus and minus symbols refer to phase indications in the equations that define these orbitals rather than electrostatic charge (in the diagram the phases are also colour coded). When the phases line up, the orbitals overlap to create a region of like phase, with the most overlapping orbitals generating the zone.
When contrasting the lowest energy molecular orbital of 1,3,5-hexatriene with benzene, pi1, the main difference in stability can be noted. There are 5 stabilising bonding contacts in the pi1 molecular orbital of 1,3,5-hexatriene compared to 6 stabilising bonding interactions in the pi1 of benzene. The ring-shaped p orbitals of benzene enable the sixth bonding interaction. The pi1 molecular orbital of benzene has a lower energy than the pi1 molecular orbital of 1,3,5-hexatriene because it has more stabilising bonding connections. As a result, benzene has an increase in aromatic stability that the acyclic 1,3,5-hexatriene lacks.
Conclusion
The real link between the carbon atoms in benzene is neither a single bond nor a double bond. Instead, each bond is a mix of a single bond and a double bond. The pi-bonding electrons in benzene are entirely free to travel around the ring. Delocalized electrons are those that are free to move between three or more atoms and are not restricted to the link between two atoms. The most effective way to demonstrate the delocalization of the electrons in benzene is to depict the compound with a ring inside the hexagon and with the hydrogen atoms known.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
