How Many Optically Active Isomers are Possible for C5H11Cl
Introduction
Optically active compounds are compounds that can rotate the plane of polarization of light. These compounds have a specific arrangement of atoms that causes them to have chirality or handedness. Chiral compounds can exist as two different mirror-image forms, known as enantiomers.
Explanation
In the case of C5H11Cl there are a total of two possible isomers that are optically active. This is because C5H11Cl has four chiral carbon atoms, and each of these carbon atoms can exist in two different configurations: one that is the mirror image of the other. Therefore, the total number of possible optically active isomers for C5H11Cl is 24 = 16.
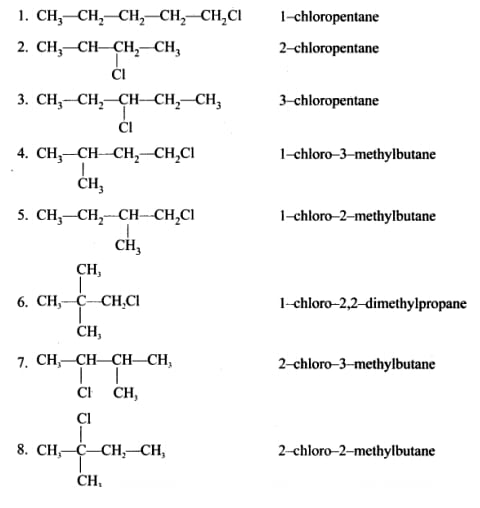
However, not all of these 16 isomers will be unique. Some of them will be the same compound, just in a different orientation. For example, one isomer may be the mirror image of another isomer, in which case they would be considered the same compound.
We must also understand what an optically active isomer means. An optically active isomer is a type of isomer that can rotate the plane of polarized light. This property is a result of the specific arrangement of atoms in the molecule, which causes the compound to have chirality or handedness. Optically active isomers can be found in many different types of compounds, including organic and inorganic compounds. In organic compounds, optically active isomers are commonly found in molecules that contain carbon atoms. These carbon atoms can exist in different configurations, which can cause the molecule to have chirality. To determine whether a compound has optically active isomers, it is necessary to examine the specific arrangement of its atoms. If the compound has chiral carbon atoms, then it is likely to have optically active isomers. These isomers can be identified by drawing out their structural formulas and comparing them to see if they are mirror images of each other.
To determine the exact number of unique optically active isomers for C5H11Cl we need to consider the specific arrangements of the atoms in the molecule. We can do this by drawing out the structural formulas for each of the possible isomers and then comparing them to see which ones are the same.
After doing this, we can determine that there are only two unique optically active isomers for C5H11Cl such that, one is the mirror image of the other. These isomers are known as enantiomers, and they are distinguished by their ability to rotate the plane of polarized light in opposite directions.
Conclusion
In conclusion, the number of optically active isomers for C5H11Cl is two. This is because C5H11Cl has four chiral carbon atoms, and each of these carbon atoms can exist in two different configurations. When these configurations are combined, there are a total of 16 possible isomers. However, not all of these isomers are unique, and after considering the specific arrangements of the atoms in the molecule, we can determine that there are only two unique optically active isomers for C5H11Cl.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
