How Many Joules is 1 Electron Volt
Introduction
1.6021766341019 Joules are equivalent to one electronvolt. In the article, we discuss the value of joules and electron volts and calculate how many joules are in 1 electron volt. Energy is measured in electron volts or eV. In the International System of Units or SI units, a joule is a derived unit of energy. A proportional relationship exists between eV and joules. The Joule equivalent value will fluctuate in the same proportion as the value of eV.
Explanation
Joules Definition
Electron Volt Definition
Let's first clarify what these units are and when they are used before beginning the conversion of an electron volt to a joule. The amount of energy that an electron gains as it is accelerated under a potential difference of one volt is measured in electron volts. Any energy that an electron gains when it is pushed across a potential difference of 1V while at rest is referred to as 1 eV.
The abbreviation eV stands for electron-volt. It is an energy unit. This symbol is specifically used in high-energy physics.
Joule Definition
The work performed by a force of one newton acting through one metre is equivalent to one joule, a unit of work or energy in the International System of Units (SI). Its name honours English physicist James Prescott Joule and its equivalent in ergs is 107, or around 0.7377 foot-pounds. The joule is equivalent to one watt-second in electrical terms, or the amount of energy released in a second by a current of one ampere flowing over a resistance of one ohm. Any of the following can also be used to define one joule: One coulomb-volt (CV) is the amount of effort needed to carry a single unit of electrical charge across a single volt of electrical potential difference. The volt may be defined using this relationship.
One watt-second (Ws) is the amount of labour needed to generate one watt of electricity for one second (compare a kilowatt-hour, which is 3.6 megajoules). The watt may be defined using this relationship. The joule bears James Prescott Joule's name. Its symbol begins with an upper case letter (J), as with every SI unit named after a person, but when written in full, it follows the rules for capitalization of a common noun; for example, "joule" is capitalised at the start of a sentence and in titles but otherwise is written in lower case.
It is equivalent to the amount of labour required to move a mass one metre in the direction of an applied newton of force. It is also the energy lost as heat when a one-ampere electric current travels for one second through a one-ohm resistance. It bears the name James Prescott Joule after the English scientist (1818–1889).
Electron Volt definition
A single electron, when accelerating from rest through an electric potential difference of 1 volt in a vacuum, acquires an amount of kinetic energy known as an electronvolt (eV). This energy unit is calculated by multiplying the charge of an electron (1.6021766341019 C) by 1 volt (1 J/C). Although not an SI unit, the electronvolt is widely used in physics and high-energy astrophysics. It is often represented with prefixes such as milli, kilo, mega, and giga (meV, keV, MeV, GeV) and is calculated as an integer multiple of the elementary charge multiplied by the voltage. The electronvolt is a standard unit in the study of particle accelerators and is frequently used in various areas of physics, including solid state, atomic, nuclear, and particle physics.
conclusion
The amount of electrostatic work is measured in electron volts (eV).
W = charge(Q) potential difference is the formula for electrostatic energy (v).
The amount of energy that an electron gains as it is propelled across a 1-volt potential difference are known as an electron volt (eV).
Electrostatic\,energy\,W = Charge\,of\,electron\times Potential\,difference\\
![]()
Charge\,of\,electron = 1.6\times 10^{^{-19}} C\\
Potential\,difference = 1\,v\\
W = 1.6\times 10^{-19}\times 1\\
= 1.6\times 10^{-19}
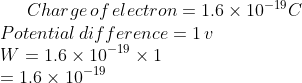
Since joule is a unit of energy.
Hence 1 eV = 1.6\times 10^{-19} J
![]()
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
