How Many Grams does an Atom of Hydrogen Weight
Introduction
The chemical element hydrogen is represented by the letter H and atomic number 1. The lightest element is hydrogen. Under normal circumstances, hydrogen is a gas made up of diatomic molecules with the formula H2. It is non-toxic, tasteless, colourless, odourless, and extremely flammable. It is an extremely light gas that can catch fire. The lightest of all gases and the most prevalent element in the cosmos is hydrogen, abbreviated H. Its atomic weight is 1.00794 and it has an atomic number of 1.
What is Molecular Weight?
The total atomic weights of the atoms in a molecule are measured by their molecular weight. To calculate stoichiometry in chemical equations and reactions, chemists employ molecular weight. M.W. or M.W. are two frequent abbreviations for molecular weight. Atomic mass units (AMU), Daltons, or a unitless expression can express molecular weight (Da).
The mass of the isotope carbon-12, which is given a value of 12 amu, serves as the reference point for defining both atomic weight and molecular weight. Because there are many carbon isotopes, the atomic weight of carbon is not exactly 12.
Calculation of a Sample Molecular Weight
The molecular formula of a substance serves as the basis for the computation of molecular weight (i.e., not the most straightforward procedure, which only includes the ratio of types of atoms and not the number). The consequences of the other bits are added after multiplying the quantity of each type of atom by its atomic weight.
A Gram Molecule of Hydrogen
A gram molecule is the number of molecules present in one mole of that substance, instead of the atoms.
Weight of one mole of Hydrogen = 1.008 g
Weight of 6.022\times 10^{23}
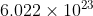 atoms of Hydrogen = 1.008 g.
atoms of Hydrogen = 1.008 g.As 6.022\times 10^{23}
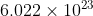 atoms of hydrogen weights, 1.008 g.
atoms of hydrogen weights, 1.008 g.1 atom of Hydrogen weighs =
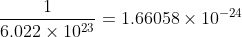 grams.
grams.Therefore, the weight of 1 atom of hydrogen is 1.66058\times 10^{-24 }
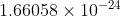 grams.
grams.
How to Estimate Molecular Weight
The size of the molecule in question affects empirical data on a compound's molecular weight. Small to medium-sized molecules' molecular masses are frequently determined using mass spectrometry. Viscosity and light scattering determine the importance of more giant molecules and macromolecules (such as DNA and proteins). For example, the hydrodynamic methods of diffusion-ordered nuclear magnetic resonance spectroscopy (DOSY), dynamic light scattering (DLS), size-exclusion chromatography (SEC), and viscometry may be applied.
Atomic Number and Isotopes
Be aware that you should use the atomic weight of the particular isotope you are working with rather than the weighted average provided by the periodic table if you are working with individual isotopes of an atom. For instance, if you are only working with the isotope deuterium and not hydrogen, you would use 2.00 instead of 1.01 for the atomic mass of the element. The difference between the atomic weight of a component and that of a particular isotope is typically relatively small, but it can be significant in some computations!
Molecular Mass vs Molecular Weight
It is sometimes used interchangeably in chemistry to refer to molecular mass as well as molecular weight. A measure of mass is a molecular mass, and an estimate of force acting on a molecular mass is a molecular weight. "Relative molecular mass" would be a better name to describe molecular weight and molecular mass as they are used in chemistry.
Conclusion
The term "molar mass" refers to the mass of one mole of a substance. The mole idea allows us to make an educated guess as to how many atoms are present in one mole of a substance. The number of atoms in the compound's molar mass can then be determined using the mole concept formula. From there, we can figure out how much one atom of the provided substance weighs.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
