Markovnikov Rule - Meaning, Definition, Applications, FAQs
In 1869, Vladimir Vasilyevich Markovnikov generalized a rule which describes the mechanism of addition reactions of protic acid to an asymmetrically substituted alkene and this rule is termed as Markovnikov rule or Markownikoff rule.
Markovnikov Rule Definition
It states that when a protic acid (specifically hydrogen halides) is added to an unsymmetrical alkene, then the hydrogen atom of the protic acid will form a bond with the doubly bonded carbon atom bearing the greater number of hydrogen atoms while the anionic part of the acid will form a bond with carbon atom which is more substituted (contain lesser number of hydrogen atoms).
- Markovnikov Rule Definition
- Mechanism of Markovnikov Rule
- Practical applications of Markovnikov rule
- What is the Anti-Markovnikov rule?
In other words, this rule can be simplified as “rich get richer” i.e., if a reaction involves the addition of atoms where the hydrogen atom and other group are added, then the doubly bonded carbon consisting of the greatest number of hydrogen atoms initially, will receive the hydrogen atoms and will get richer in H atoms. An example of Markovnikov rule is as follows:
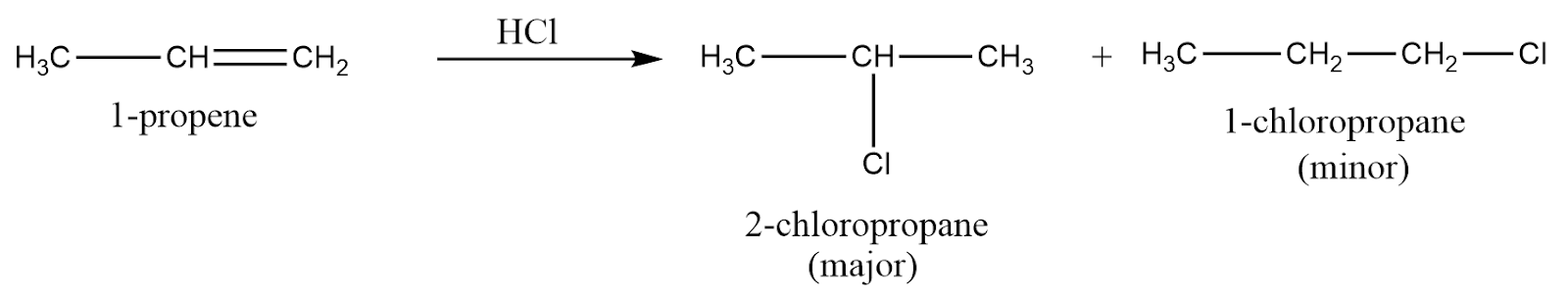
It is clearly observed from the reaction that two products are obtained from addition reaction of unsymmetrical alkene i.e., a major product which is formed according to Markovnikov rule and a minor product which is formed according to Anti-Markovnikov rule (we might discuss this rule later in this article). Thus, these reactions are regioselective because there is a preference for forming one regioisomer over the other during the reaction.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Mechanism of Markovnikov Rule
For better understanding of the mechanism, let us consider the example which we illustrated earlier i.e., addition reaction of hydrogen chloride (hydrochloric acid) with propene. The mechanism of Markovnikov rule involves several steps which are as follows:
Step-1: Dissociation of hydrogen chloride (HCl) into its respective ions:
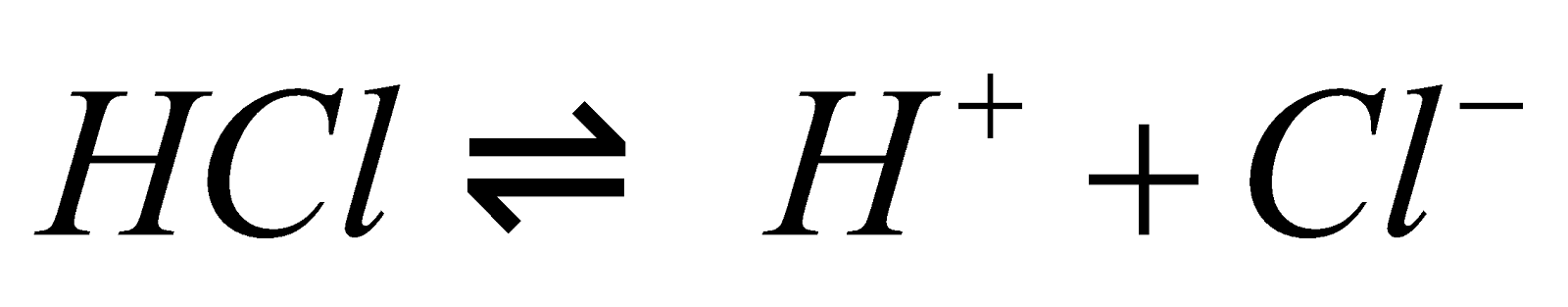
Step-2: Protonation of alkene takes place and most stable carbocation is formed as an intermediate after the reaction as follows:
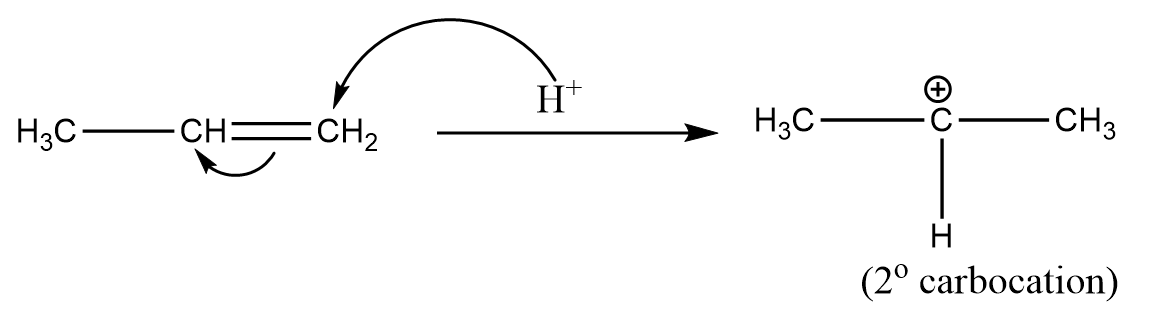
Thus, the intermediate involved during the addition of HCl to propene is carbocation and as the 2" carbocation is relatively more stable than 1º carbocation because of the greater inductive effect, so its formation is preferred over the formation of primary carbocation.
Step-3: The chloride ion acts as a nucleophile and attacks the carbocation due to which deprotonation of carbon atom takes place as per following reaction:
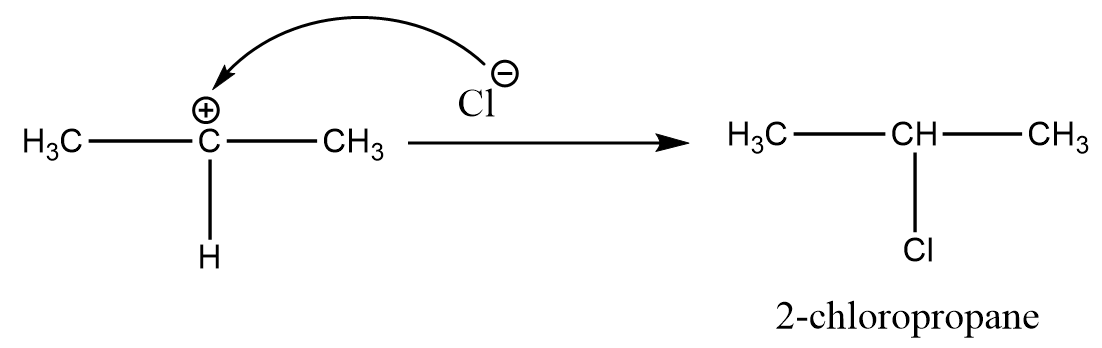
Since secondary carbocation is preferred over primary carbocation, so the reaction will yield 2-chloropropane as the major product.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
- NCERT notes Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Practical applications of Markovnikov rule
1. Formation of halohydrin (in alcohol and water)-
This reaction involves breaking off the pi bond of alkene and forming a halohydrin (halo means halogen and hydrin means hydroxyl group) in its place. This reaction takes place in water and yields a product which follows the Markovnikov rule. An example for this reaction is as follows:
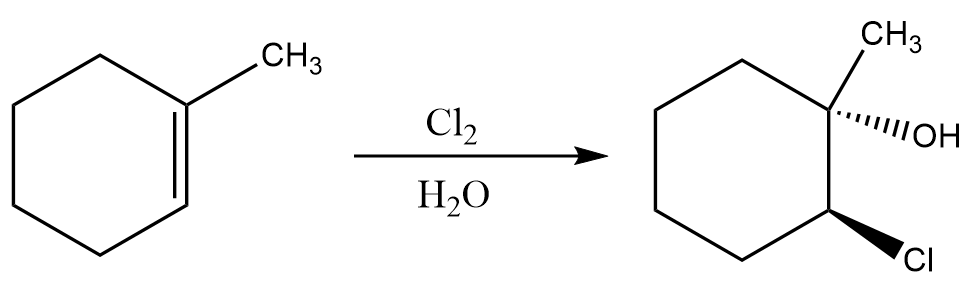
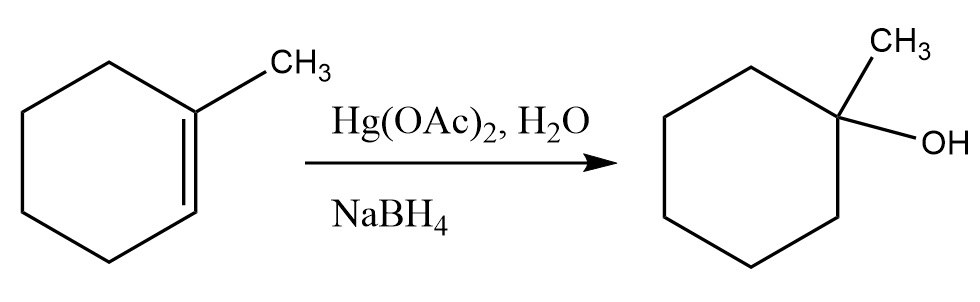
2. Oxymercuration Demercuration-
It is a reaction which results in the Markovnikov additions of hydrogen and a hydroxyl group on the alkene and forming an alcohol. The intermediate involved in the reaction is mercurinium ion instead of carbocation and proceeds in the presence of Hg(OAc)2, H2O (for oxidation) and NaBH4 for reduction. An example for this reaction is as follows:
Related Topics Link
3. Acid catalysed hydration reactions-
This reaction results in the Markovnikov addition of a hydrogen and a hydroxyl group across an alkene forming an alcohol. Intermediate in this reaction is carbocation and no stereospecificity is associated with this reaction. An example of this reaction is hydration of propene in the presence of dilute sulphuric acid and the reaction is represented as follows:
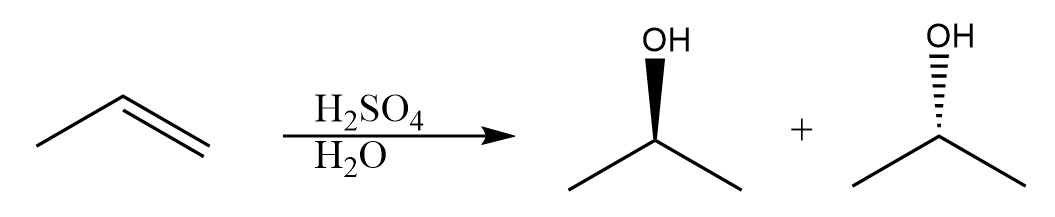
These reactions are involved in the industrial usages of Markovnikov’s rule and are widely used in a variety of chemical processes.
What is the Anti-Markovnikov rule?
This rule describes the regiochemistry where the electronegative part of the reagent is bonded to a less substituted carbon atom rather than the more substituted carbon atom. This rule is followed only when hydrogen bromide HBr reacts with unsymmetrical alkenes in the presence of hydrogen peroxide and free radical is the intermediate involved in this process. An example for this reaction is given as follows:
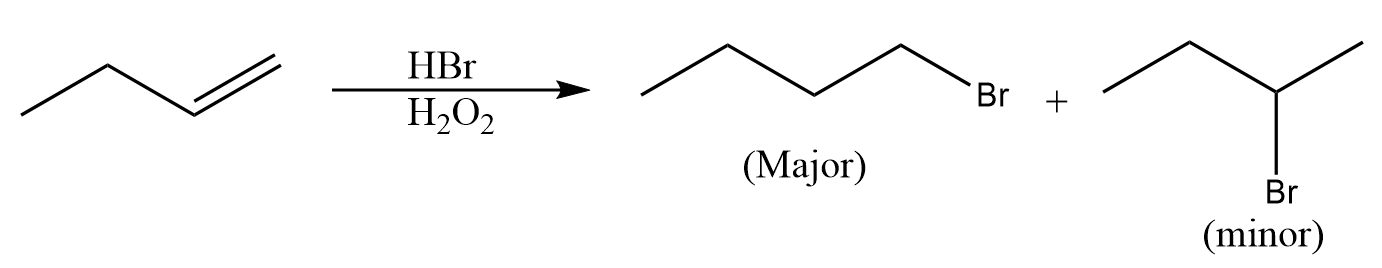
Practical applications of Anti-Markovnikov rule:
Hydroboration-oxidation-
It is a type of anti-Markovnikov reaction in which a hydroxyl group form a bond with the less substituted carbon atom and it is a two-step reaction which includes a hydroboration step in which alkene is treated with BH3 in the presence of non-polar solvent like THF(tetrahydrofuran) and an oxidation step in which hyperoxide ion reacts to form respective alcohol. An example for the reaction is given as follows:
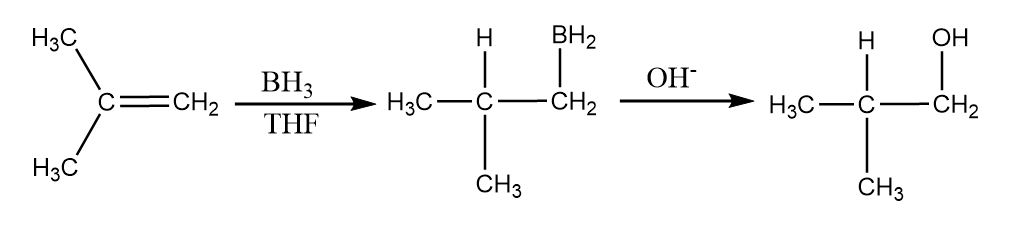
Thus, we can conclude that Markovnikov addition basically involves protonation of a double bond to give a cation on a more substituted carbon atom, which traps a nucleophile to form final saturated products. The attack of nucleophile control over two important aspects in the formation of a product i.e., regioselectivity and stereoselectivity.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Markovnikov rule states that the electronegative part of the adding molecule will form a bond to that doubly bonded carbon atom which is highly substituted.
The reaction intermediate involved in the Markovnikov addition reaction is carbocation.
Markovnikov rule explains that in addition reaction of alkenes, the proton is added to the carbon atom which consist of greatest number of hydrogen atoms while Anti-Markovnikov rule (peroxide effect) explains that in addition reaction of alkenes, the proton is added to the carbon atom which has the least number of hydrogen atom associated to it.
In the presence of hydrogen bromide HBr and peroxide like H2O2 only, the product will be formed according to anti-Markovnikov rule because halogens other than bromine does not undergo free radical addition to alkenes as the abstraction of proton is an endothermic reaction and the new bond formed after addition cannot compensate the amount of energy provided. In case of bromine, the abstraction of proton and bromine radical is exothermic and thus, reaction is feasible.
A. the Aufbau principle
B. the nucleophilicity of bromide anion
C. the acidity of HBr
D. the relative strength of carbocations
Ans. Option (D) is correct.
Also Read
10 Jul'25 01:56 PM
02 Jul'25 08:03 PM
02 Jul'25 05:10 PM
02 Jul'25 05:06 PM
02 Jul'25 04:57 PM
02 Jul'25 04:54 PM
02 Jul'25 04:52 PM
02 Jul'25 04:51 PM
02 Jul'25 04:50 PM


