Benzene Hexachloride - Uses, Properties, Structure, Preparation, FAQs
Benzene hexachloride is an organic compound which is an isomer of hexachlorocyclohexane with the chemical formula C6H6Cl6. This compound is also given the name lindane or hexachloride. It is a solid that is colorless and with an odor of slightly musty. This compound is also an organochlorine compound that has a wide application in the agricultural field and pharmaceutical industry.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
This compound is also given the notation BHC for simplicity. Normally benzene compounds do not undergo addition reactions fast so the reaction required for the preparation of benzene hexachloride needs some extra outside stimulant which is light-induced. And thereby the addition of chlorine to benzene gives many isomers which are alpha, beta, and gamma of which the isomer that is useful as an insecticide also has much application is called the lindane or otherwise it is called gammaxene that is the gamma isomer of benzene hexachloride has much use.
Benzene hexachloride was first discovered in 1825 by Faraday. The Dutch chemist Lindane discovered or isolated the gamma isomer in 1912 hence the name Lindane is given to the gamma isomer of the compound benzene hexachloride. Whereby the gamma isomer isolated has shown that it possesses insecticidal properties and is more toxic than many other compounds.
The structural difference between alpha, beta, and gamma is in their orientation of the chlorine atoms concerning the ring of carbon atoms. The volatility of the gamma isomer of BHC is more than DDT. Mergamma A is a compound used for seed treatment and this compound contains 1 % Mercury and 20 percent lindane.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Preparation of benzene hexachloride
Benzene hexachloride can be prepared by many methods, some of the methods for the preparation of benzene hexachloride are described below
- In the presence of sunlight and in the absence of oxygen that is the reaction is done in a closed atmosphere where the benzene reacts with chlorine in presence of sunlight to form benzene hexachloride and a substitution catalyst may be used to increase the rate of this reaction.
- Benzene hexachloride can be prepared by the photochlorination method where the isomers of hexachloride are formed, therefore to extract the gamma isomer the reaction mixture is treated with methanol or acetic acid. Whereby the alpha and the beta isomers of benzene hexachloride dissolve easily and we can easily separate gamma isomers from the reaction mixture. The following reaction shows the preparation of benzene hexachloride from benzene and chlorine.
C6H6+3Cl2 hv→ C6H6Cl6
Properties of benzene hexachloride
Benzene hexachloride is a chemical compound with the molecular formula C6H6Cl6 That means it contains 6 chlorine atoms in addition to the benzene ring. The molecular weight or molecular mass of this organic compound is 290.814 grams per mole with a density of 1.89. The melting point of benzene hexachloride is 113 degrees Celsius and the boiling point is 323 degrees Celsius.
BHC Structure
The image shown below describes the structure of benzene hexachloride that with the chemical formula C6H6Cl6. Benzene hexachloride is a Gamma isomer and it has a serious effect on human beings; it is a toxic compound. From the below structure of benzene hexachloride, we obtain that it is a compound that contains 6 carbon atoms, 6 hydrogen atoms, and 6 chlorine atoms that means the double bonds of benzene has been substituted with the chlorine compounds.
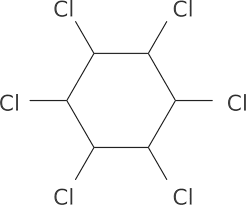 Structure of BHC
Structure of BHC
Related Topics Link, |
Toxicity of benzene hexachloride
It is a highly toxic compound when it comes in contact with skin, eyes, etc. And also when it is inhaled the effects are very much. It has a serious effect on the nervous system so that further it may lead to the death of the individual who has suffered from the toxicity of benzene hexachloride. Lindane or gamma benzene hexachloride has been found that gets accumulated in the food chain whereby from animals to human beings and also from marine life.
Exposure to lindane may cause acute poisoning and which further affects the nervous system. Benzene hexachloride is a neurotoxin that causes toxicity by interfering with the functioning of neurotransmitters. It is also shown that serious exposure has been adversely affected liver functions in human beings. This also reported that it has effects on the kidneys of human beings and therefore it is a type of carcinogen as well. BHC insecticides are highly toxic organochlorine compounds.
In addition to the effects on human beings, it can also affect the environment as it is an organic pollutant. When this Pollutant persists in the environment the causes serious effects on the environment and also it will enter the food chain. When Lindane is present in soil it gets broken down into less harmful substances by the process of fungi and bacteria but this process is relatively slow so the use of this compound must be reduced thereby our environment can convert it into a less harmful substance. There are so many reports showing that in some countries the use of this gammaxene is reduced.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
- NCERT notes Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Uses of BHC
- Gamma benzene hexachloride commonly known as limited is used as an insecticide.
- It is present in some creams and shampoos for the treatment of lice infection.
- It has application in the pharmaceutical industry.
- It can be used for the treatment of scabies.
- Benzene hexachloride is used as a weedicide therefore it is also used as a fertilizer.
- Benzene hexachloride is used against domestic cockroaches.
Gamaxin powder uses
Lindane is known as gammaxene also called BHC, which has a wide application in the field of the agricultural industry and pharmaceutical industry. It is used as an insecticide and pesticide in the agricultural sector. But due to the high toxicity of this compound, some countries reduced its production. It is used in pharmaceuticals for the production of shampoos and creams for the infections caused by lice. And also for the treatment for the disease scabies, is a skin disease. Gammaxene powder used in horticulture. Gammaxene has now replaced the place of DDT due to its best results.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Benzene hexachloride( BHC).
C6H6Cl6.
A type of polyhalogenated organic compound with the chemical formula C6H6Cl6 is BHC (Benzene hexachloride). It is obtained by the chemical reaction in presence of sunlight with benzene and chlorine. It is a white crystalline solid also called lindane and gammaxene with a wide application in the agriculture and pharmaceutical industry. It is widely used as an insecticide and pesticide. But due to high toxicity, the use of this compound in the agricultual sector has been reduced by many countries. The most insecticidal isomer of BHC is the gamma isomer that is called lindane. There are many other isomers for BHC. The one with the most extensive application is its gamma isomer.
1,2,3,4,5,6 Hexachlorocyclohexane.
Lindane.
Also Read
02 Jul'25 08:03 PM
02 Jul'25 05:10 PM
02 Jul'25 05:06 PM
02 Jul'25 04:57 PM
02 Jul'25 04:54 PM
02 Jul'25 04:52 PM
02 Jul'25 04:51 PM
02 Jul'25 04:50 PM
02 Jul'25 04:46 PM

