Stephen Reaction Mechanism - Imine, Benzaldehyde, DIBAL-H, FAQs
What is Stephen Reaction:
Defining Stephen reaction, Stephen reaction is also called as Stephen aldehyde synthesis or Stephen Reduction Reaction. It was first discovered by a chemist Henry Stephen. This reaction is organic redox reaction, whose end product is an aldehyde. The reaction is also named as Stephen reduction reaction.
- What is Stephen Reaction:
- Stephen Reaction Mechanism:
- Imine:
- Benzaldehyde:
- Mendius Reaction Mechanism:
- DIBAL-H:
Stephen Reduction Reaction:
In Stephen reduction reaction when methyl nitrile reacts with Hydrochloric acid and tin chloride it produces the iminium salt. This iminium salt further quench with water to give aldehyde as product. We also get some by product in this reaction such as ammonium chloride or ammonia. The aldehydes produced in the reaction is aromatic or aliphatic in nature. The iminium salt produced as intermediate products is not stable so further quenching is required in Stephen reduction reaction.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Explain Stephen Reaction:
In Stephen reaction mechanism when Tin(II) chloride with hydrochloric acid than it produces tin tetra chloride and two hydrogen ions. As in this case of Stephen reaction the Tin(II) stage gets converted to form Tin(IV) by the removal of two electrons from it. Here to show the Stephen reduction reaction we take methyl cyanide or phenyl cyanide as alky nitrile or aryl nitrile in which nitrogen is more electronegative compare to carbon.
So it is found that nitrogen is partially negative and carbon is partially positive. Pie bond present in cyanide, in which one is broke down and due to that electrons move to nitrogen atom and simultaneously two electrons which were released in the reaction will accepted by carbon of cyanide atom. Therefore both atom carries negative charge on it.
Nitrile to Aldehyde Mechanism:
Here we are showing an example of Stephen reaction. In Stephen reaction acetaldehyde is being produces by the action of methyl cyanide. The action of nitrile to aldehyde is shown below in the equation.
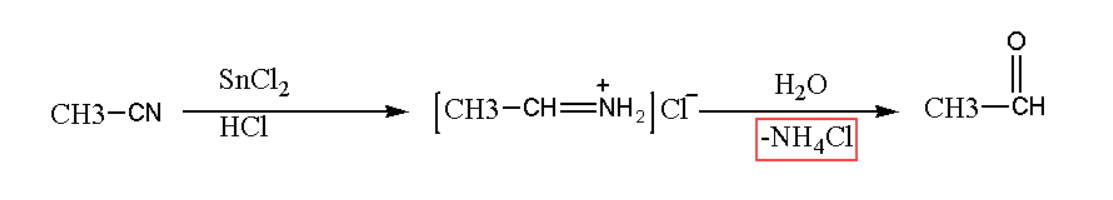
In the above Stephen reaction mechanism the intermediate product was formed and this product is imine, which is formed by the action of nitrile when undergoers reduction with stannous chloride and hydrogen chloride in ethyl acetate solvent. This intermediate product of Stephen mechanism will further hydrolyse to yield the required aromatic or aliphatic aldehyde.
Stephen Reaction Mechanism:
Steps followed by the Stephen reaction mechanism:
Step1. In the first step of Stephen reaction the gaseous hydrogen chloride is being added to the nitrile, which reacts to give its correlated salt as shown below in the reaction.
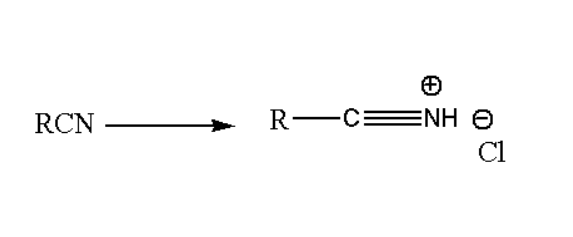
Step2. In the next second step of the Stephen reaction single electron transfer occur in Stannous(II) chloride to form its salt. The reaction is:
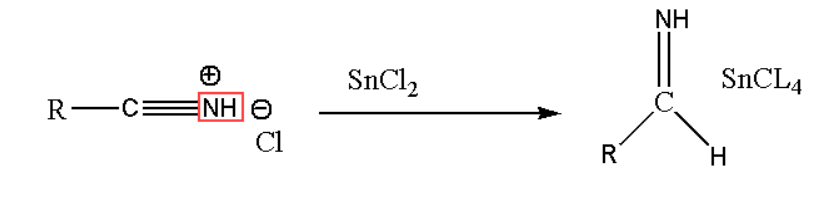
Step3. In the third step of Stephen reaction the obtained salt gets precipitates. The salt is aldimine tin chloride shown in the reaction.
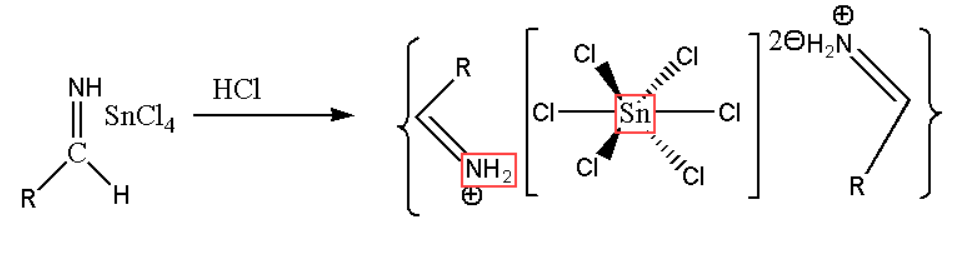
Step4. In this step hydrolysis of the obtained salt is done which gives us amide. This end product is the r5equired aldehyde as shown below:
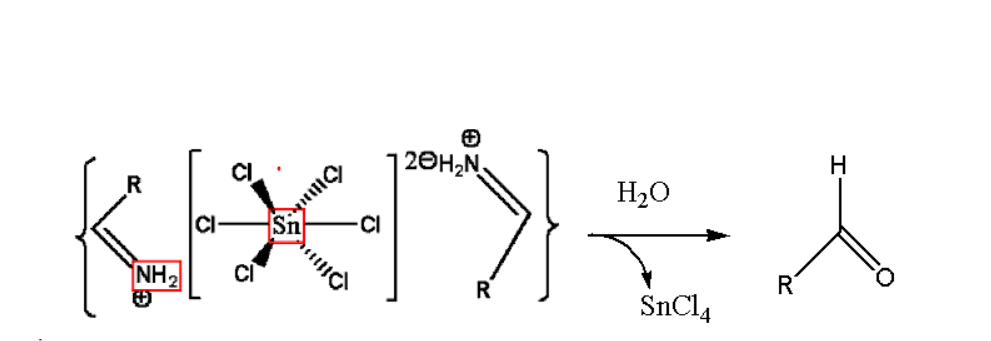
By the above steps we get the end product as aldehyde in Stephen reaction mechanism. One important key point is to note that instead of using aliphatic nitrile, aromatic nitrile is more efficient to be used. Substitutes that are added which improves the electron density also improves the formation of salt of tin chloride. Amide chloride formation can also be promoted by electron withdrawing substitutes.
NCERT Chemistry Notes:
Imine:
An Imine is defined as a chemical compound that contains the atom of carbon and nitrogen with double bond. Imine is the functional group used in many reactions of organic chemistry. The Nitrogen atom of Imine can be attached to any organic group or with hydrogen easily. The term Imine first used in 1883 by German Chemist named Albert Ladenburg.
Imine Group:
Imines will easily undergo to hydrolysis with their correlated compounds of amine or carbonyl. Imines may get precipitated when reacted under aldehydes and ketones. In the reaction mechanism of heterocycles imines are widely used as the intermediate product. Formation Imines ca be possible by the condensation of primary amines and aldehydes. Synthesis of Imines can be predicted as shown below.
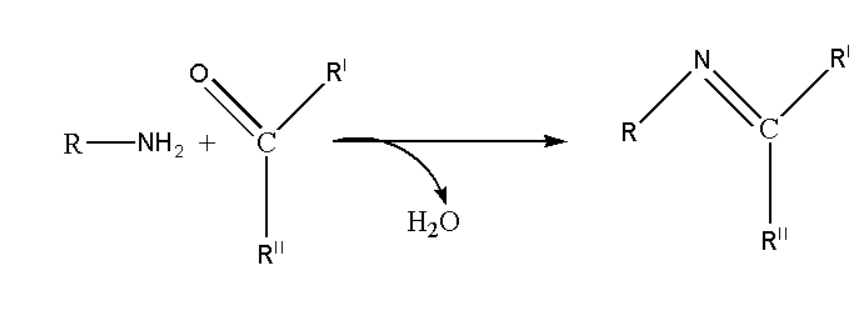
The important point is Iminium cation is functional group in which nitrogen has fourth bond, which gives iminium cation to positive charge.
Related Topics Link, |
Benzaldehyde:
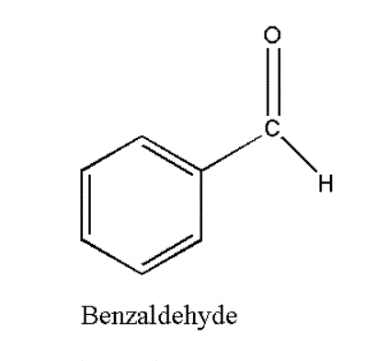
Benzaldehyde is the common organic compound which is consisted of benzene ring with formyl substituent in it. It is mostly used as for industrial purposes and is the simplest aromatic aldehyde. Benzaldehyde formula is ![]() . Benzaldehyde density is 1.04 g/
. Benzaldehyde density is 1.04 g/![]() . According to IUPAC nomenclature it is named as Benzenecarbaldehyde. Benzaldehyde is found as colourless liquid. Its smells like almond. It is the primary component of bitter almon oil.
. According to IUPAC nomenclature it is named as Benzenecarbaldehyde. Benzaldehyde is found as colourless liquid. Its smells like almond. It is the primary component of bitter almon oil.
Extraction of Benzaldehyde is possible from number of other natural resources. For the other bakery products or flavoured cakes it is used as flavoring agent. It can also be used in the cosmetic products, pharmaceuticals to plastic additives etc. Benzaldehyde can also be used as bee repellent. Benzaldehyde is safe to use as it does not produces non-carcinogenic compounds which is used in foods and cosmetics. Structure of Benzaldehyde is shown below:
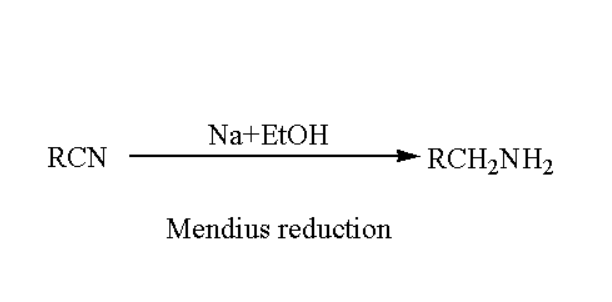
Mendius Reaction Mechanism:
In the Mendius reaction mechanism organic compounds are involved which contains the cyanide group. Formation of ammines occur by the action of alcohol and alkali metals.In the Mendius reduction reaction ethanolic sodium is used as reagent in it.Basically we can define the Mendius reaction as the reduction of alkyl and aryl cyanides to produce amines in the presence of nascent hydrogen is Mendius reduction recation. When nitrogen atom are attached to carbon atom such compounds are called Nitriles compound. In this triple bond is present in between carbon and nitrogen.
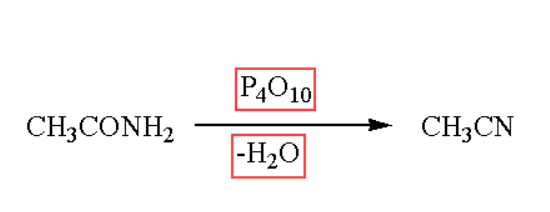
The formation of nitriles occur by dehydration of amides which means when amides are heated, it gets dehydrated to form nitriles. Dehydration process occur in the presence of phosphorous oxide.The reaction of converting to nitriles by the process of dehydration is as follows:
The obtained nitrile is further reacted with sodium atom, and it undergoes to reduce in the presence of alcohol. Such procedure yields the primary amine. And this reduction reaction of nitriles to ammines is so called Mendius Reduction reaction mechanism.
The reduction will proceeded as follows:
DIBAL-H:
Diisobutylaluminium hydride is so called DIBAL-H which is a reducing agent.This compound is found originally as co-catalyst and are known as organoaluminium compound.The compound is used as polymerization of alkenes.The DIBAL can be prepared by the use of heat, which means heating up triisobutylaluminium to get beta-hydride elimination.The DIBAL compound is basically used in reduction for converting the carboxylic acids.The DIBAL is electrophilic reducing agent so it quickly reacts with electron-poor compound.DIBAL. It is found Colourless. It is more efficiently used in place of lithium hydride and it also helps in reducing nitrioles to aldehyde so DIBAL reduces the acids as well.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 Haloalkanes and Haloarenes
- NCERT notes Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
Reaction of DIBAL-H With Cyanide:
For the formation of aldehyde and ketone, it requires strong reducing agent. Electrophillic reducing agent works well in such situation where we convert cyanide to aldehyde and ketone. The reaction of such reducing agent with cyanide is as follows:
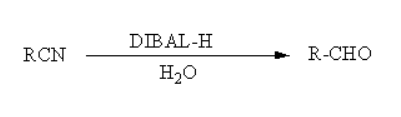
The DIBAL-H works good as Lewis acid and has a aluminium atom in it. The electron pair donated by the nitrile group and can be accepted readily by this Lewis acid. Formation of Imine occurs in this reaction when the hydrogen ion of DIBAL-H is attached with carbon atom of cyanide. Now after the formation of imine as intermediate hydrolysis is done to form aldehyde. For the conversion of cyanide to ketone we use different reagent such as Grignard reagent. This is how we convert Cyanide to aldehyde.
Also check-
Frequently Asked Questions (FAQs)
Ethyl cyanide is also called as propionitrile or propanenitrile. It is the organic compound and simply a aliphatic nitrile. The compound is found as colourless liquid and are water soluble. It is as similar to acetonitrile but have slightly higher boiling point in comparison.
Quenching chemistry is rapid cooling of any substance or any compound. Cooling of compound can be done with the process known as hydrolysis.In very short time of interval it freezes the reaction and prevents it from decomposition.
Methyl aldehyde is also known as formaldehyde which is a naturally occurring oragic compound with pungent odour. The compound polymerises to form paraformaldehyde, therefore it is stored as in aqueous solution. This is the simplest form of aldehyde with relation to formic acid.
The density of Benzaldehyde is 1.04g/cm3.
Also Read
24 Jul'25 03:53 PM
02 Jul'25 08:03 PM
02 Jul'25 05:10 PM
02 Jul'25 05:06 PM
02 Jul'25 04:57 PM
02 Jul'25 04:52 PM
02 Jul'25 04:51 PM
02 Jul'25 04:50 PM
02 Jul'25 04:46 PM


