Benzene (C6H6) - Definition, Discovery, Structure, FAQs
Benzene is the parent member of the hydrocarbon family which is composed of a highly unique unsaturated structural unit C6H6. C6H6 is a chemical formula of Benzene.
Discovery of Benzene:
The majority of hydrocarbons possessed a sweet and pleasant odor and hence they were named aromatic compounds. Benzene was discovered by Michael Faraday of the Royal Institution Back in 1825.
What is benzene?
Michael Faraday was the first person to come up with the name “bicur-buret of hydrogen” for this aromatic compound which is now known as Benzene. Michael was successful in isolating benzene from the compressed gas of whale oil. In the wake of Benzene's curiosity in 1834, Eilhardt Mitscherlick heated benzoic acid with calcium oxide which led to the successful synthesis of benzene.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
C6H6COOH+CaO ∆→ C6H6+CaCO3
The Molecular formula of benzene was quite shocking at that time because this was for the first time when a hydrocarbon was discovered with an index of hydrogen deficiency of. Whereas, the other compounds had twice the number of Hydrogen. This meant that C6H6 would be a highly unsaturated compound. However, the properties of benzene were highly unexpected as it was expected to show properties of unsaturated compounds.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
C6H6 Structure:
In 1865, August Kekulé was the first person to put forward the structure of benzene which satisfied the four bonds of Carbon and one bond of hydrogen. The structure has six carbons and six hydrogens bonded through alternating single and double bonds.
In the beginning, Kekulé suggested that the two forms of benzene are in resonance with each other and this equilibrium is established so rapidly that it is not possible to separate the two forms. However, now we know that no such equilibrium exists. The unique stability of Benzene is due to aromaticity. Even though the compound is saturated it gives a substitution reaction in place of addition reactions.
Stability of Benzene-
Resonance in benzene is a major reason for the high stability of benzene.
For an unsaturated compound, it is quite unexpected to not show any addition reactions. Benzene is a compound which undergoes substitution reactions but does not undergo addition reactions.
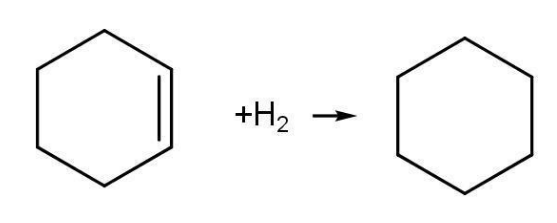
Cyclohexene cyclohexane ∆H= - 119.5 kJmol-1
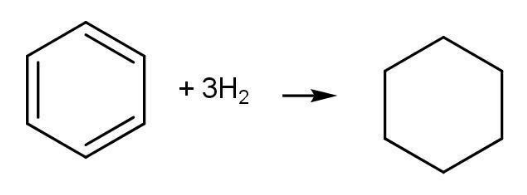
Cyclohexatriene cyclohexane ∆H= -119.5 ✕ 3= -358.5 kJmol-1
Experimental value of enthalpy of hydrogenation of benzene
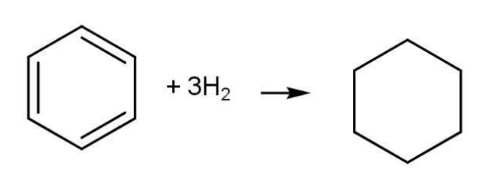
Benzene cyclohexane ∆H= -208.1 kJmol-1
Enthalpy of hydrogenation for Cyclohexene having one single bond is 119.5 kJ mol-1. For benzene, the enthalpy of hydrogenation containing three double bonds should be three times the enthalpy of hydrogenation of cyclohexene i.e -358.5 kJ mol-1
However, experimental value for enthalpy of hydrogenation of benzene is -208.1 kJ mol-1
This means that benzene is more stable than the hypothetical cyclohexene molecule by 150.4 kJ mol-1. This value depicts the Resonance energy in Benzene. The difference between the energy of hypothetical cyclohexatriene and that of benzene is called Resonance energy of benzene.
Kekulé structure of benzene:
The stability of benzene can be explained by the resonance hybrid structure of benzene. Number of resonating structures of Benzene are two and one resonance hybrid of the two resonating structures. Actual Benzene molecule is comparatively more stable than either of the resonating structures given by kekule.
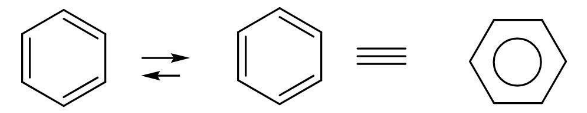
Kekulé structures of benzene Resonance hybrid
It is the resonance structure of benzene.
Bond Length of benzene C-C bond-
All C-C bonds in benzene are equal in length due to resonance. Bond length of the carbon-carbon single bond is 139 pm and this value is the same for all the bonds. ∏ e-1 charge density is spread over a large area which leads to delocalization. Delocalization leads to a decrease in energy. This resonance energy stabilizes the benzene ring.
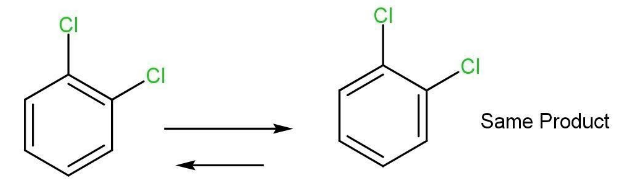
If the two resonating structures of the benzene truly existed then there would be two isomers of 1,2-dichlorobenzene. However, it is not true. Only one such structure exists, double bonds keep switching between C=C double bonds and hence only one product is formed.
Related Topics link, |
Orbital picture of benzene:
According to molecular Orbital theory of benzene, all the bond angles of carbon atoms in the benzene ring are 180⁰ which indicates the sp2 hybridization of all the six carbon atoms. All the carbon atoms are sp2 hybridized except one orbital which remains unhybridized. The six electrons of the pi bond give the electronic configuration of the ground state.
Out of the three orbitals of carbon, two orbital overlap axially with neighbouring carbon atoms to form a sigma bond and the third hybrid orbital of carbon overlaps with the orbital of hydrogen atom to form carbon and hydrogen sigma bonds. The unhybridized orbitals on each of the carbon atoms form a pi bond through sidewise overlapping. There is a pi cloud on the benzene ring due to continuous rings above and below the carbon atoms .
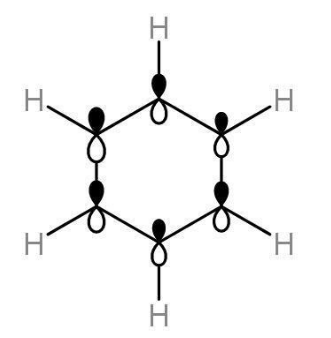
Fig1 -Unhybridized 2p orbital of benzene ring
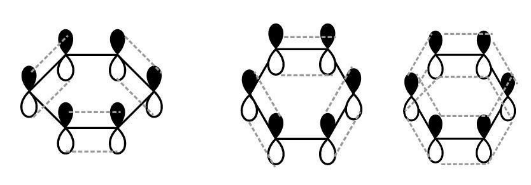
Fig2 -Sidewise overlap of the Unhybridized p orbitals of benzene ring
Following are some of the Physical Characteristics of Benzene-
Smell of benzene | Aromatic |
| Density of benzene | 0.87 gm/cm3 |
| Color | Colorless |
| Boiling Point | 80.1⁰C |
| Point group | D6h |
| Flash Point | 12⁰F |
Chemistry of Benzene and its Derivatives-
- Chlorobenzene-
Chlorobenzene can be synthesized from benzene in the presence of a Lewis acid such as AlCl3 or ferric halide at a temperature between 310-320 K
- Benzene Sulphonic acid-
-SO3H group can be substituted with the hydrogen atom via Sulphonation. Benzene is heated with sulfuric acid at 330K.
- Acetophenone-
A very famous method to prepare acetophenone is by treating benzene with ethanoyl chloride or ethanoic anhydride in the presence of lewis acid. This reaction is famously known as Friedel Craft Acylation.
- Nitrobenzene-
Substituting the Hydrogen atom with the nitro (NO2) group in the presence of sulphuric acid yields nitrobenzene. Attacking species is an electrophile NO+2
- Toluene-
Benzene with CH3 is known as toluene. When an alkyl halide reacts with Benzene in the presence of Lewis acid such as anhydrous aluminium chloride toluene is formed and this reaction is famously known as Friedel- Craft alkylation.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
- NCERT notes Class 11 Chemistry Chapter 13 Hydrocarbons
Polynuclear Hydrocarbons-
Hydrocarbons containing two or more than two benzene rings are known as polycyclic aromatic compounds. Some common examples of such compounds are anthracene, phenanthrene, naphthalene. Most of the polynuclear hydrocarbons are carcinogenic in nature and are also toxic.
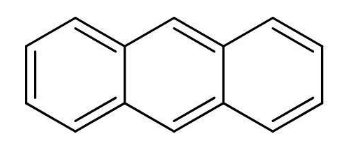 Anthracene
Anthracene
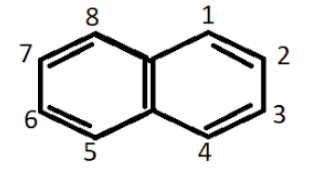 Napthalene
Napthalene
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
In order for an aromatic compound to be aromatic it has to fulfil the certain criteria-
Planarity- Molecules should be planar so that delocalization of pi electron density can occur. If the ring of the compound is planar only then this delocalization can occur.
Delocalization - Molecules must contain a cyclic pi electron cloud above and below the ring which is formed by the overlap of p orbitals. This ensures the delocalization of electron density.
Huckel rule- The molecule must contain (4n+2)∏ electrons where n= 0,1,2,3.. so on.
Benzene is a planar molecule which has delocalized electron density and also obeys Huckel’s rule as it contains a total of 6∏ electrons where n=1
From the orbital picture of Benzene the carbon atoms of the benzene ring are sp2 hybridized and one p orbital is unhybridized. Two hybridized orbitals form a sigma bond with the neighbouring hybridized orbital of carbon atoms and one hybrid orbital of carbon atom overlaps with the s orbital of Hydrogen atoms. As a result there is a pi electron cloud spread over the six carbon atoms.
This makes benzene a plane and flat molecule and electron density is delocalized over a larger area which increases the stability of the benzene ring.
Conjugate base of benzoic acid is benzoate.
C6H6 chemical name is benzene.
Also Read
11 Mar'25 06:34 PM
11 Mar'25 06:27 PM
11 Mar'25 06:25 PM
11 Mar'25 05:54 PM
11 Mar'25 05:27 PM
05 Mar'25 05:23 PM
05 Mar'25 05:18 PM
05 Mar'25 04:59 PM
19 Feb'25 07:36 PM
19 Feb'25 07:33 PM

