Isomers of Butane - Definition, Types, Structure with FAQs
Isomerism-The molecules having the same number and same types of atoms have the same formula, but have different physical and chemical properties.
Types of isomerism in butane
Constitutional isomers (Based on connectivity)
These are the isomers having the same chemical formula but the atoms or groups of atoms differ in their connectivity.
There is a possibility that butane can have two different structures based on connectivity. There can be straight chain butane as well as branched chain butane. Both these structures of butane fulfill the valency of the carbon atom and thus form four bonds. There are 13 total numbers of covalent bonds in butane as well as isobutane.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Types of isomerism in butane
- Structural isomers of butane
- Isomers of butene
- Structural isomers of C4H10O
- Isomers of butyne
The name isobutane came from the fact that it is a constitutional isomer of butane. The structural unit of the ‘iso’ group is the carbon atom bonded to hydrogen and two -CH3 groups. This is also known as chain isomerism. These are the isomers which differ in skeletons of carbon atoms.
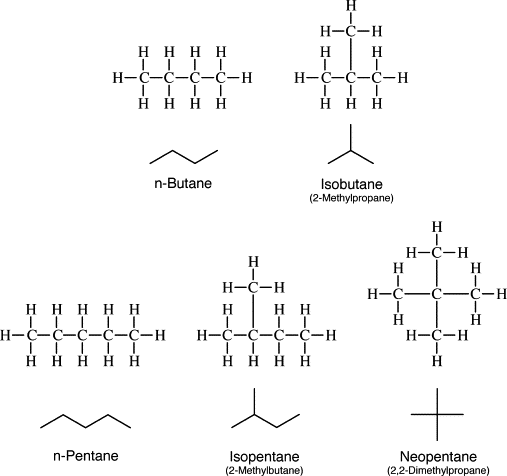
Figure 1) n butane structure
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Conformational isomers (Based on rotation around sigma bond)
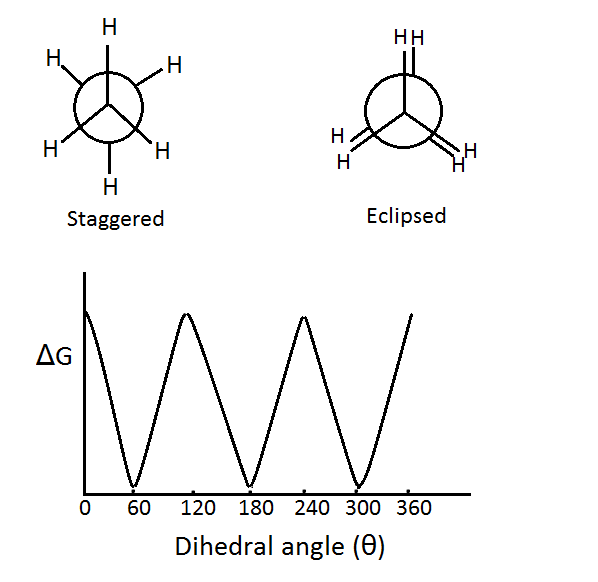
Different spatial arrangements of atoms in molecules can be obtained due to free rotation around sigma bonds. All these arrangements are known as conformations. Carbon-Carbon bond in butane is a sigma bond therefore; rotation around the carbon-carbon single bond is possible. This rotation around the C-C single bond can occur without causing any effect on the overlap of orbitals.
Large number of spatial arrangements is possible due to this rotation. However, this rotation is not totally free. An energy barrier of about 1-20 kJ mol-1 has to be crossed to overcome the torsional strain. This torsional strain arises due to the repulsive forces between electron pairs. Two types of conformations are possible-
Eclipsed conformation- In this conformation the groups around the sigma bond are directly behind those of the other. A conformation can either be fully eclipsed or partially eclipsed. According to the same difference, the energy of a conformation can increase or decrease.
Staggered Conformation- In this conformation the groups around the sigma bond are staggered with respect to each other. Staggered conformation is of two types, anti and gauche conformation.
Four conformations are possible for butane, namely anti conformation, Gauche or skew conformation, eclipsed conformation, and fully eclipsed conformation. When the rotation takes place about the C2-C3 bond, the conformation with the two methyl groups opposite to each other (or 180⁰ away from each other) is called anti conformation and is maximum staggered.
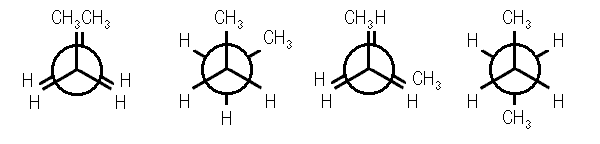
Figure 3) Anti conformation of butane
With a rotation around the C2-C3 at an angle of 60⁰, this conformation becomes eclipsed; further rotation about an angle of 60⁰ will give skew conformation.
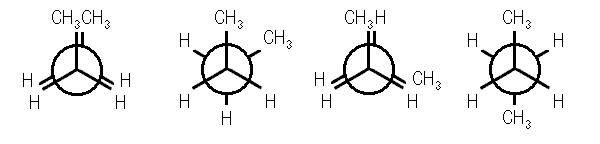
Figure 4) eclipsed conformation of butane
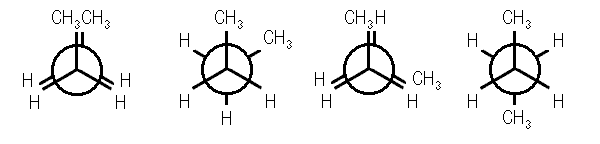
Figure 5) gauche conformation of butane
This conformation can also be staggered, however the methyl groups will be comparatively closer. Hence, there will be repulsions between the electron pairs which will lead to an increase in the energy of gauche conformation by 3.8 kJ/mol. A fully Eclipsed conformation is the most unstable conformation because the methyl groups are closest to each other. This fully eclipsed conformation has the highest energy and 19 kJ/mol more energy than the anti-conformation.
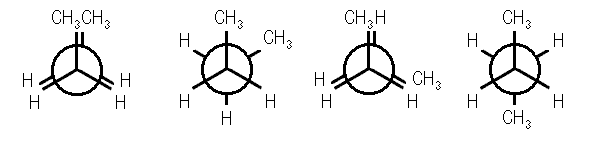
Figure 6) fully eclipsed conformation of butane
Structural isomers of butane
There are two possible isomers of butane.
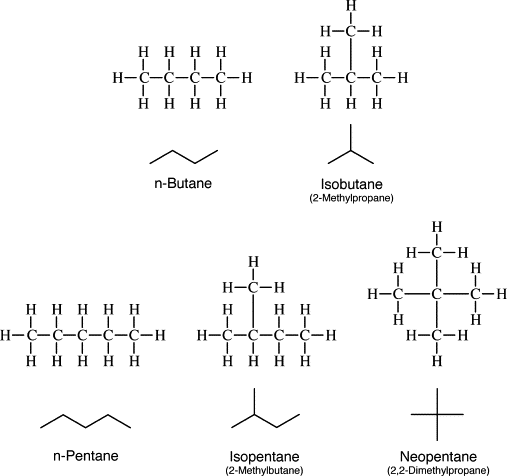
Figure 7) n butane or neo butane structure
N butane formula: C4H10
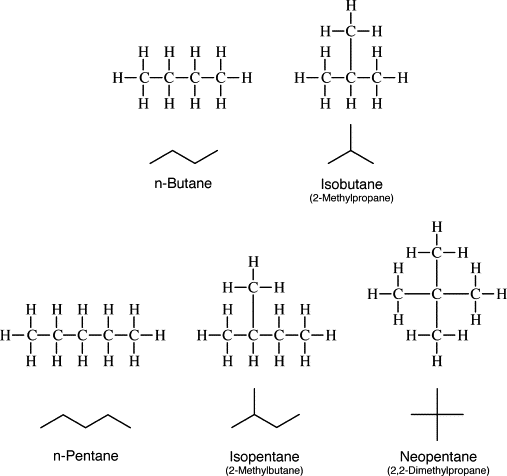
Figure 8) isobutane structure
Isobutane formula: C4H10
IUPAC name of isobutane is 2-methylpropane. That means 2 methylpropane structure formulas are the same as isobutane structural formulas.
Both isobutane and n butane are C4H10 isomers.
To understand isomerism better, let’s examine more compounds that show isomerism.
Isomers of butene
There are four possible isomers of butene.
Butene molecular formula: C4H8
Butene structural formula and C4H8 IUPAC name is discussed below:
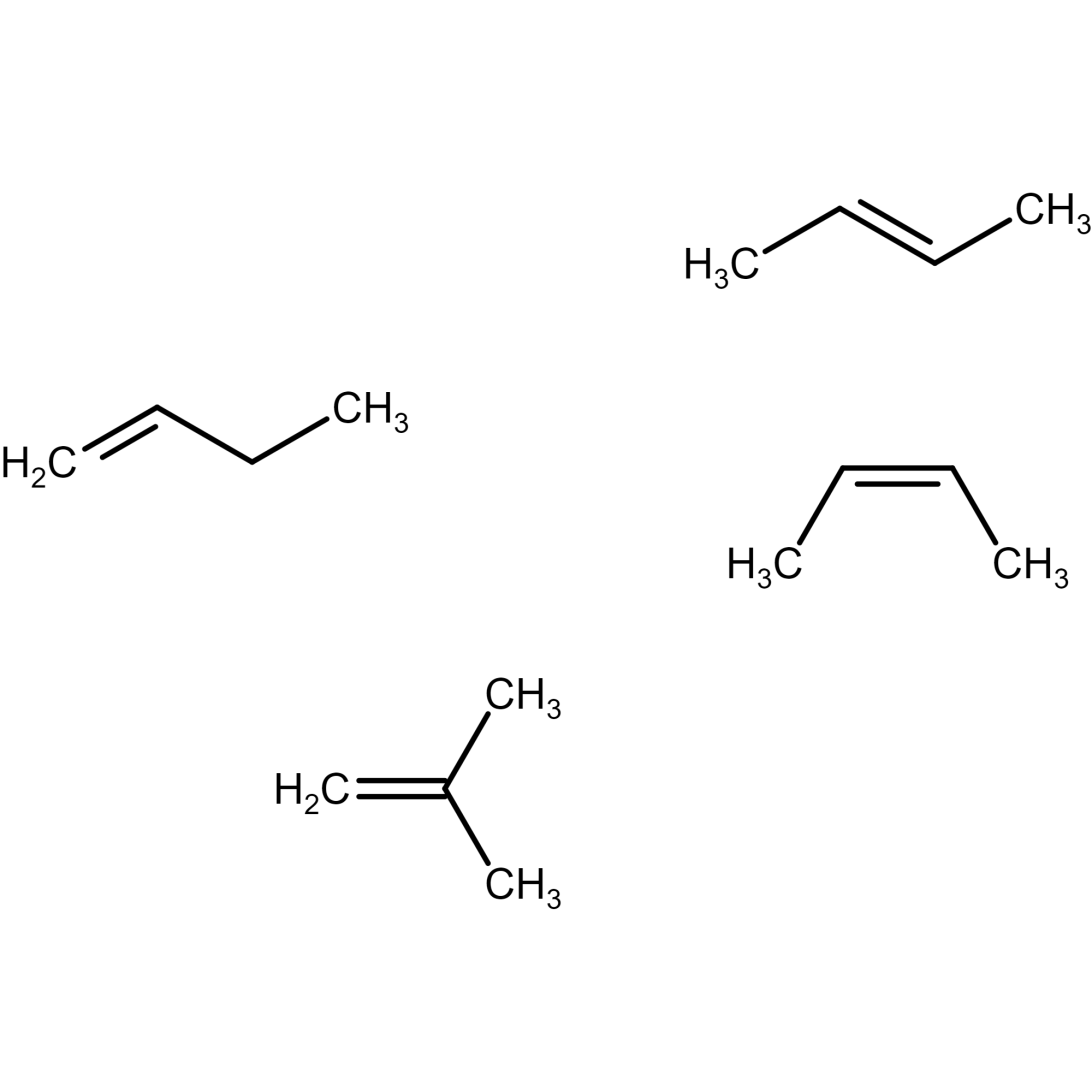
Figure 9) but-1-ene structure
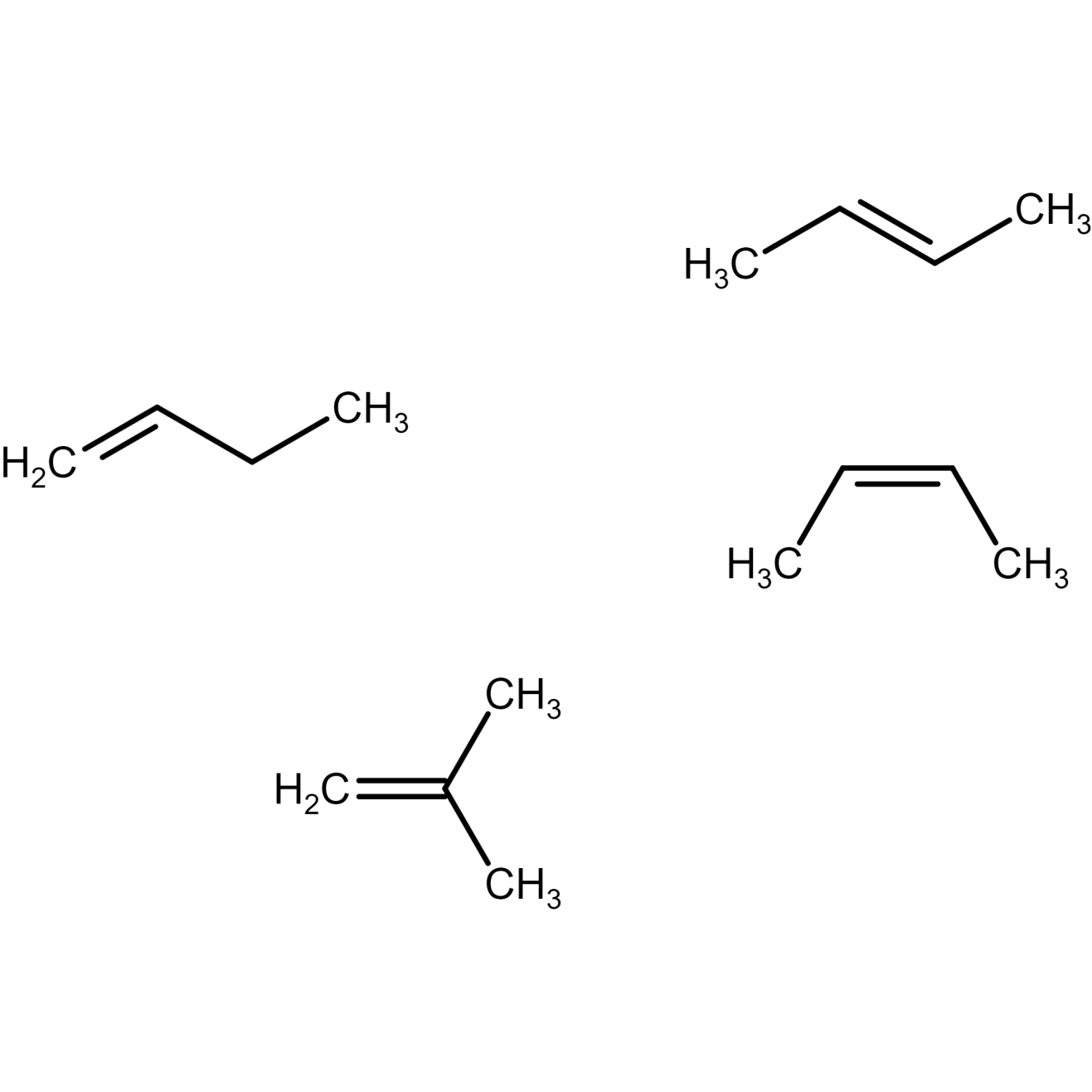
Figure 10) Trans-2- butene structure
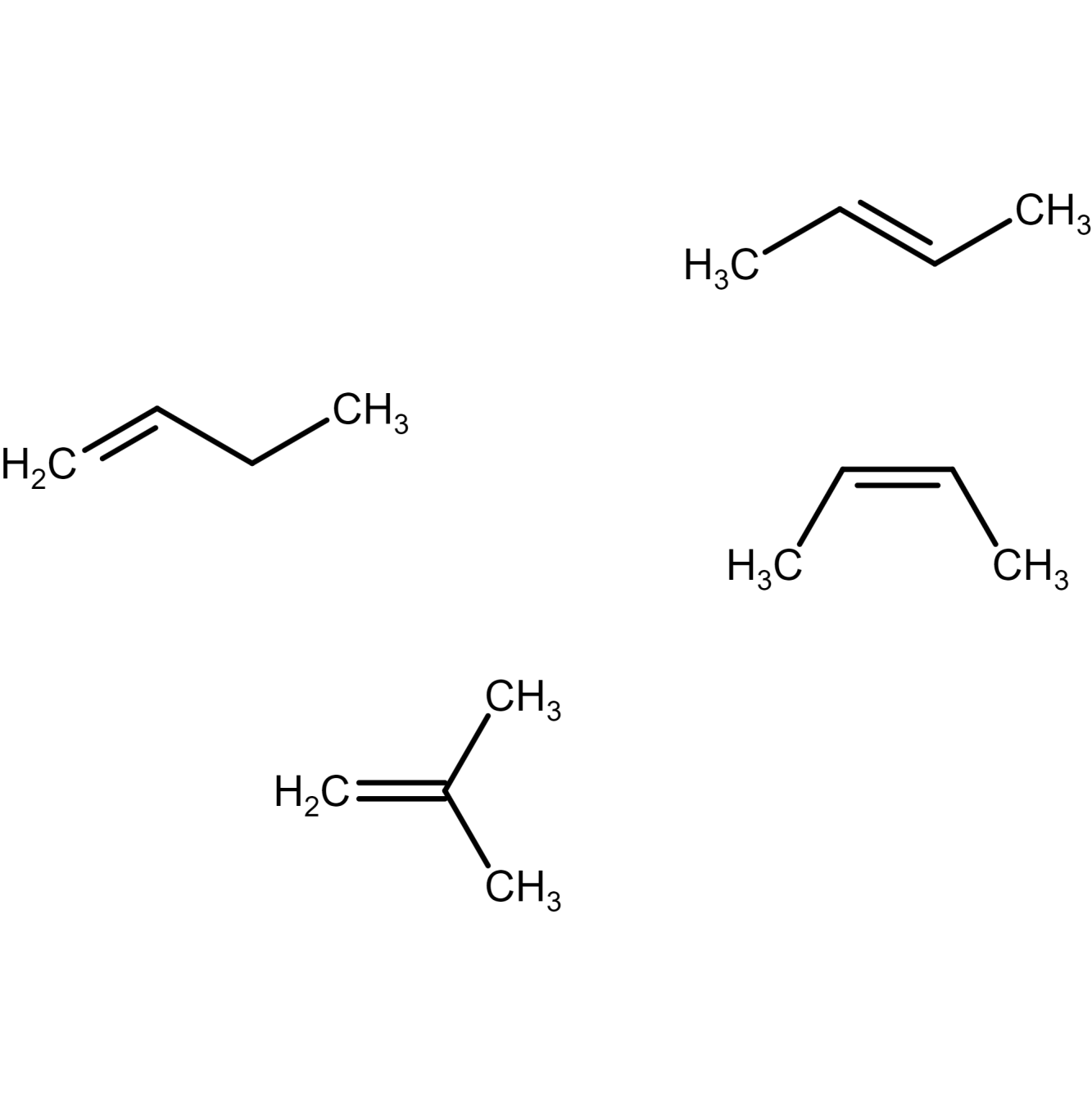
Figure 11) cis-2- butene structure
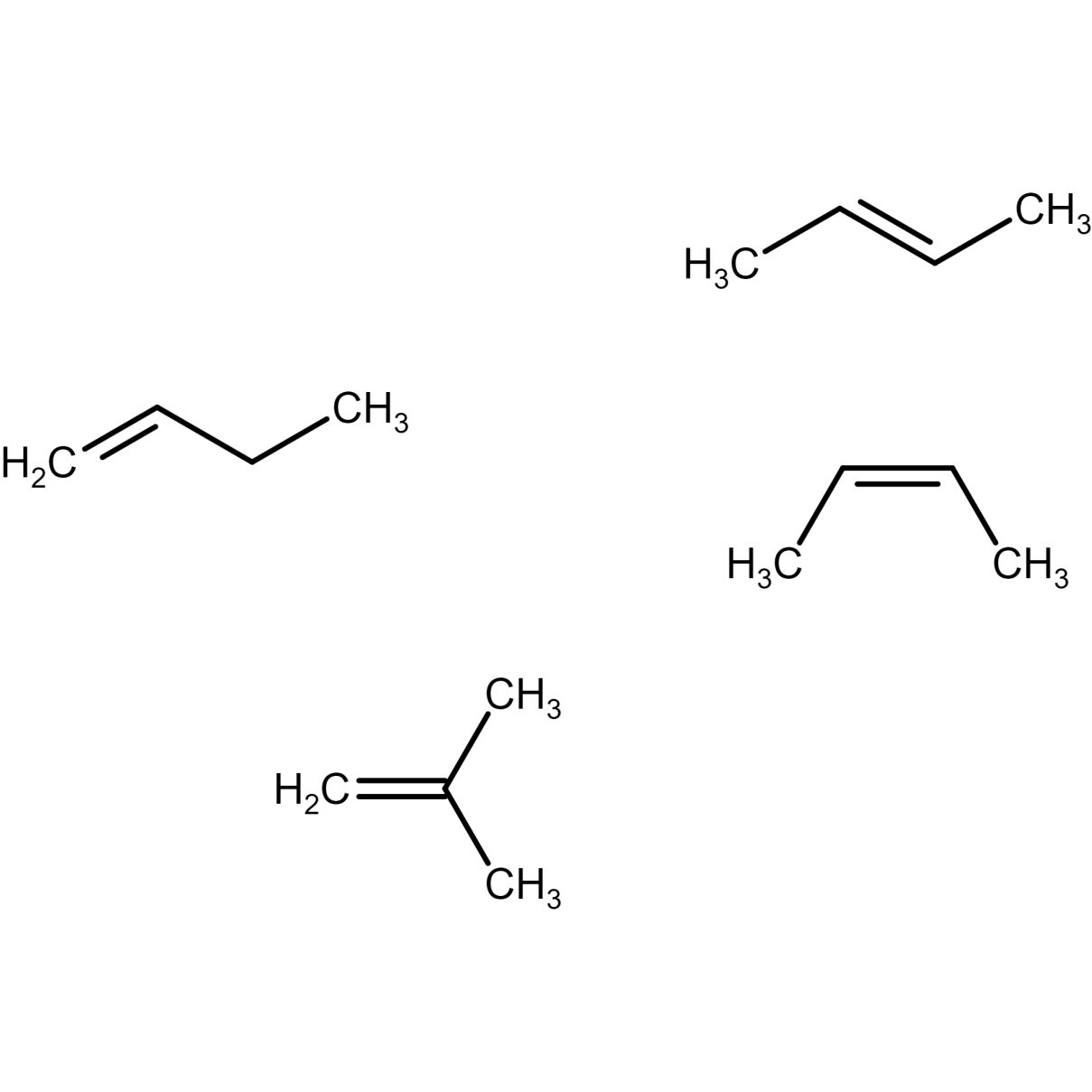
Figure 12) isobutene structure
Where, cis-2-butene and trans-2-butene are geometric isomers of 2 butene. Here, but-1-ene and but-2-ene (both cis and trans) are position isomers of butene. , but-1-ene and but-2-ene (both cis and trans) and isobutene structure are structural isomers of butene.
Understanding the isomerism in but-2-ene-
Butene structure has four carbon atoms in which two carbon atoms are connected through a double bond. Just like how conformational isomerism was possible due to free rotation around the carbon-carbon sigma bond. Another type of isomerism is possible as a result of restricted rotation around a carbon-carbon double bond called geometrical isomerism.
Structural isomers of C4H10O
The compound C4H10O can show seven types of isomers excluding optical isomers.
ALCOHOLS:
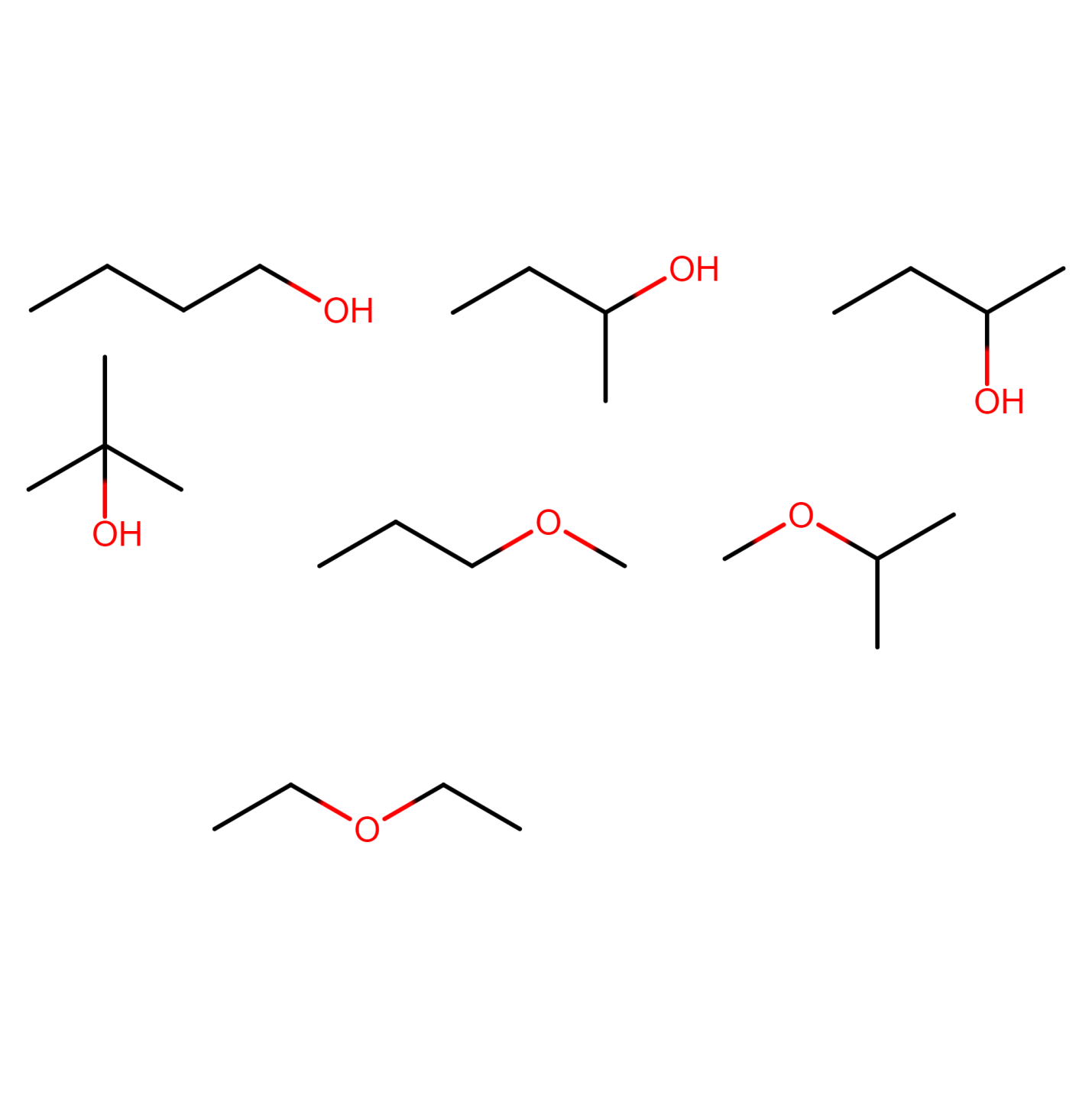
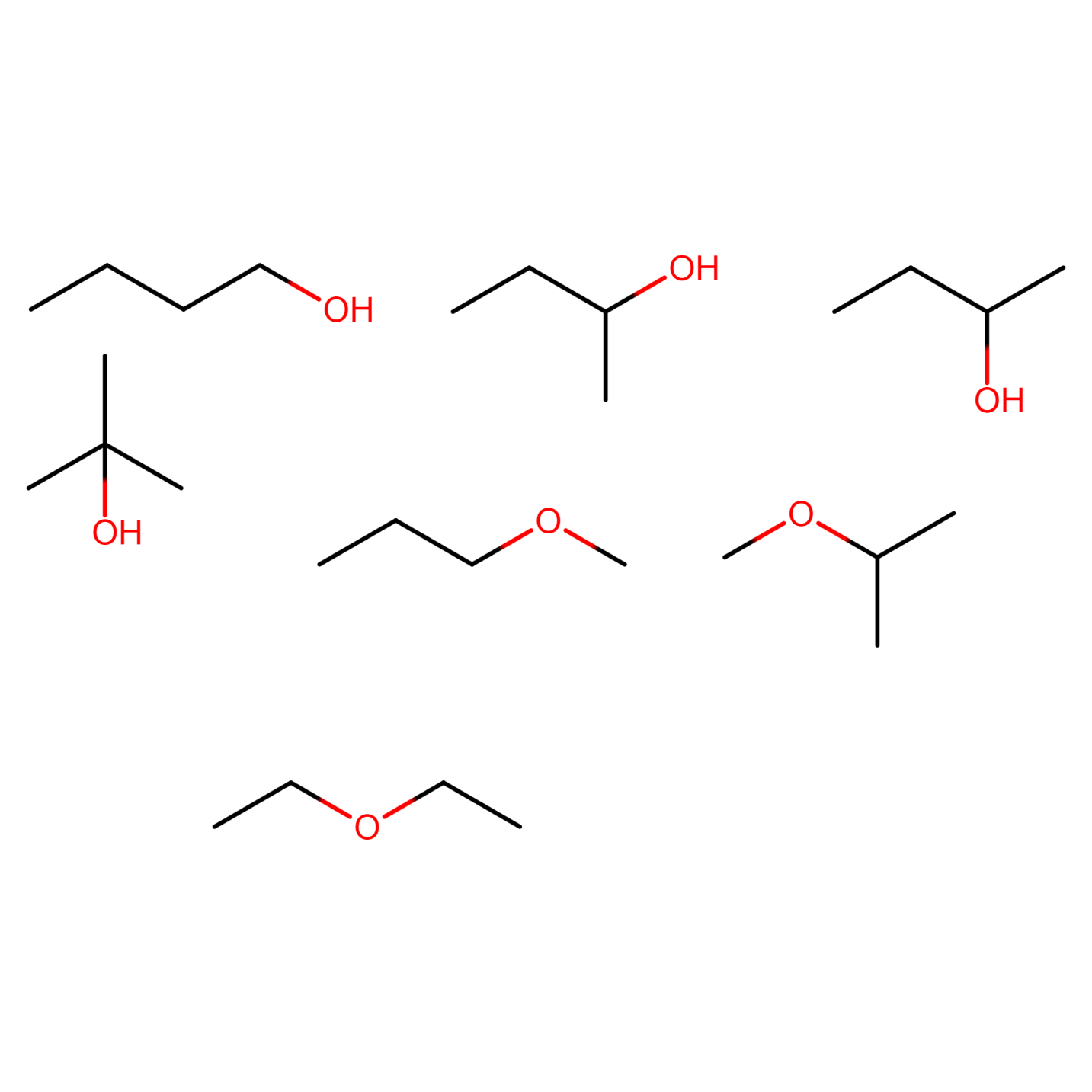
Figure 18) 2-methylpropan-1-ol
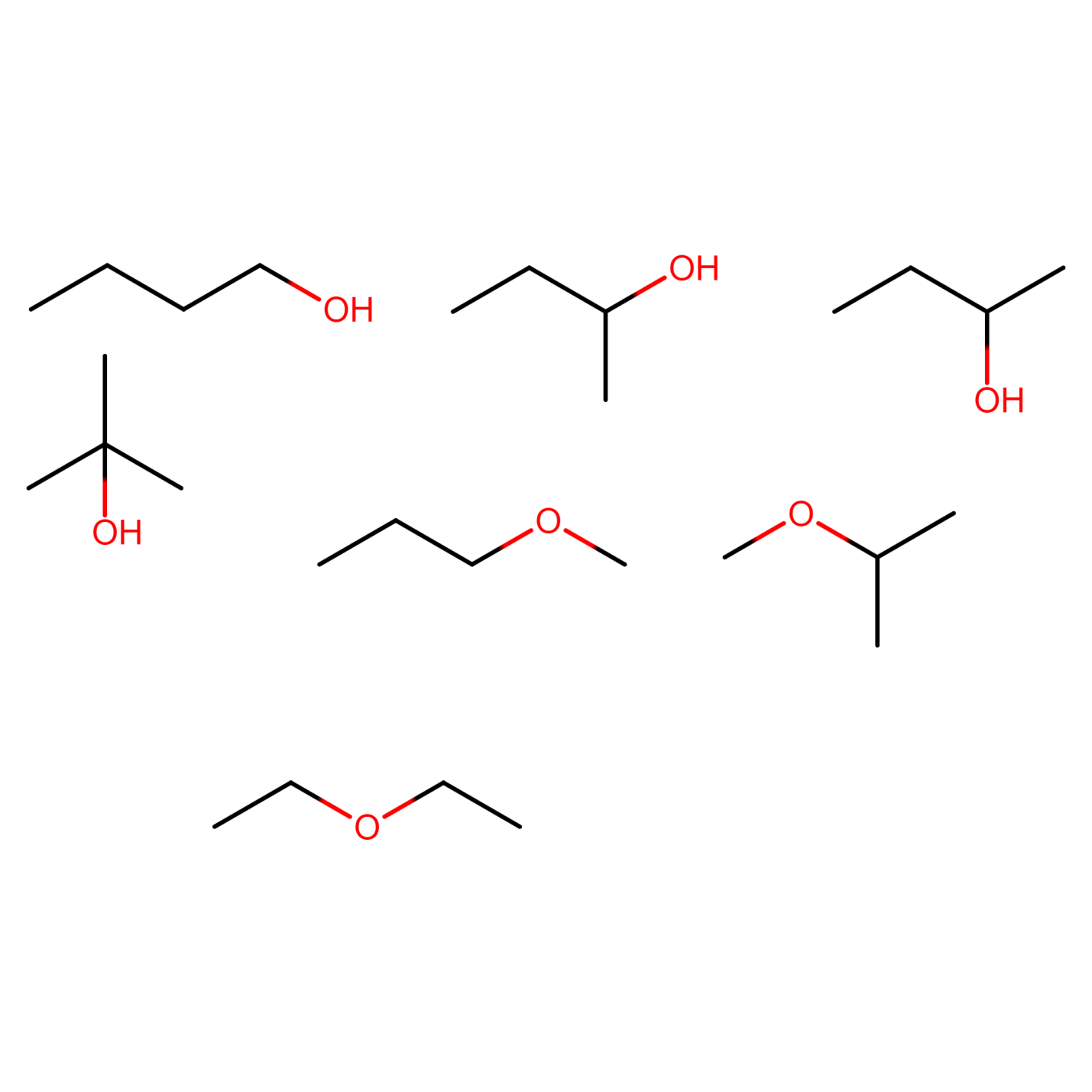
Figure 19) butan-2-ol
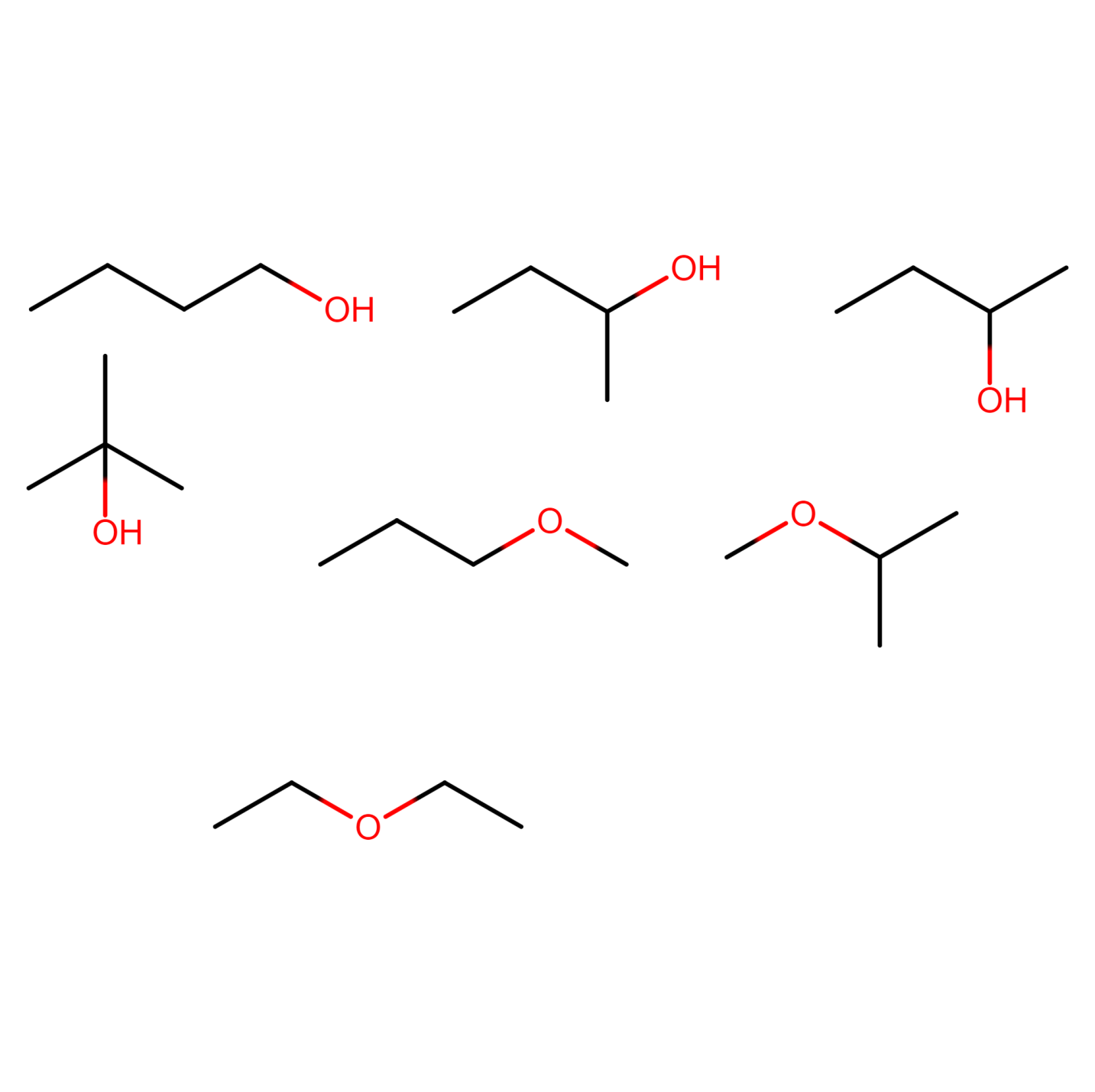
Figure 20) 2-methylpropan-2-ol
ETHERS:
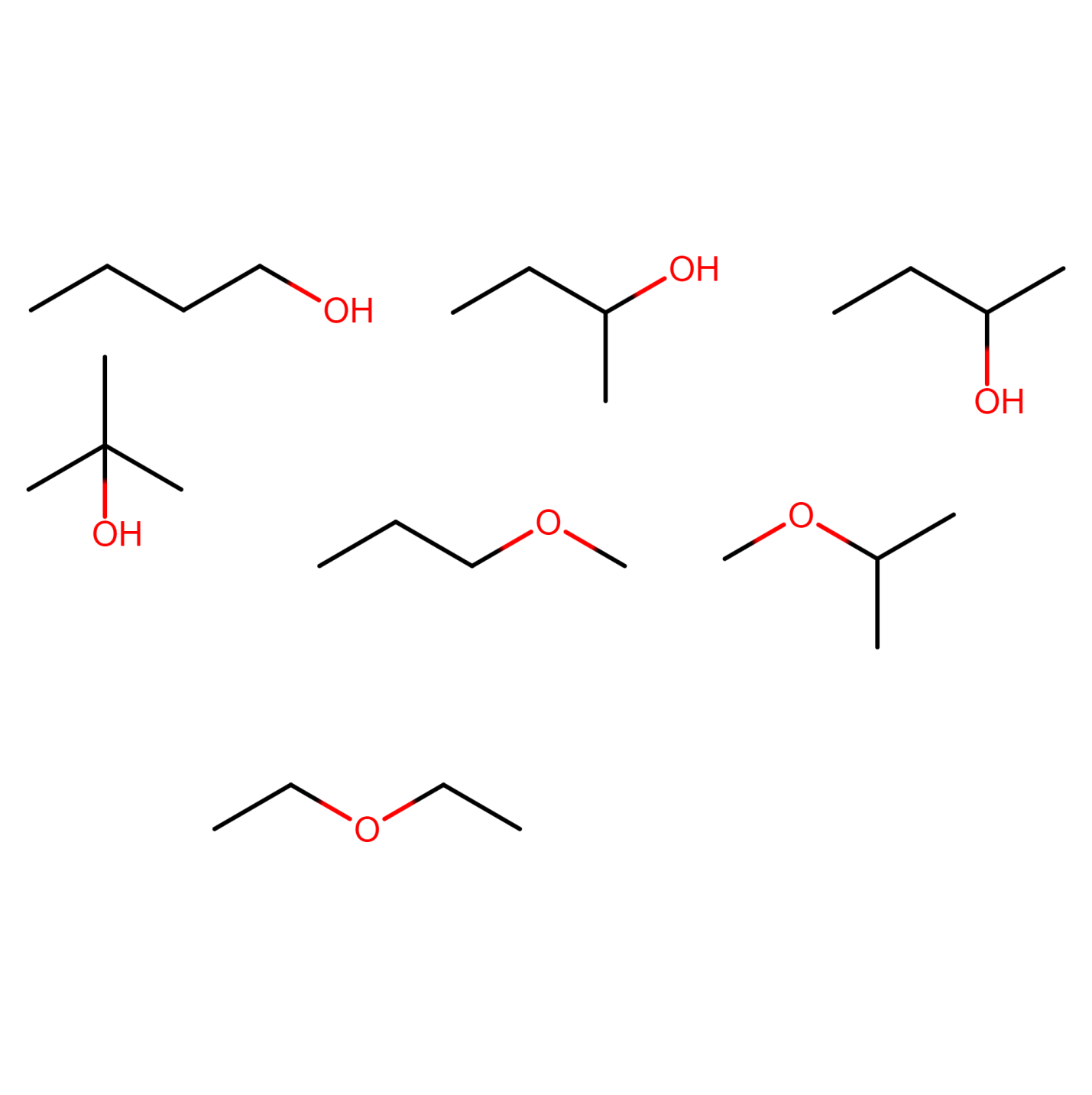
Figure 21) 1-methoxypropane
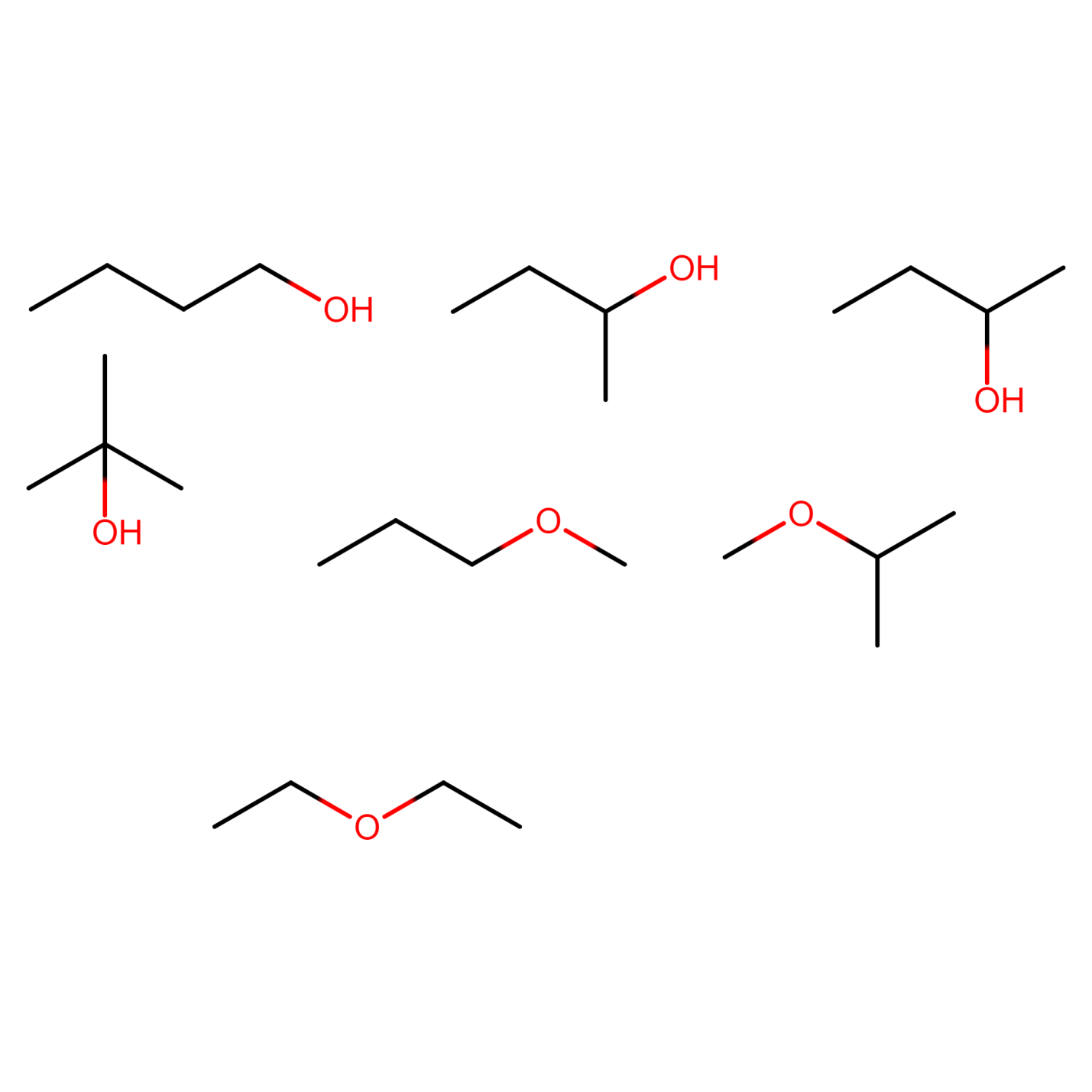
Figure 22) 2-methoxypropane
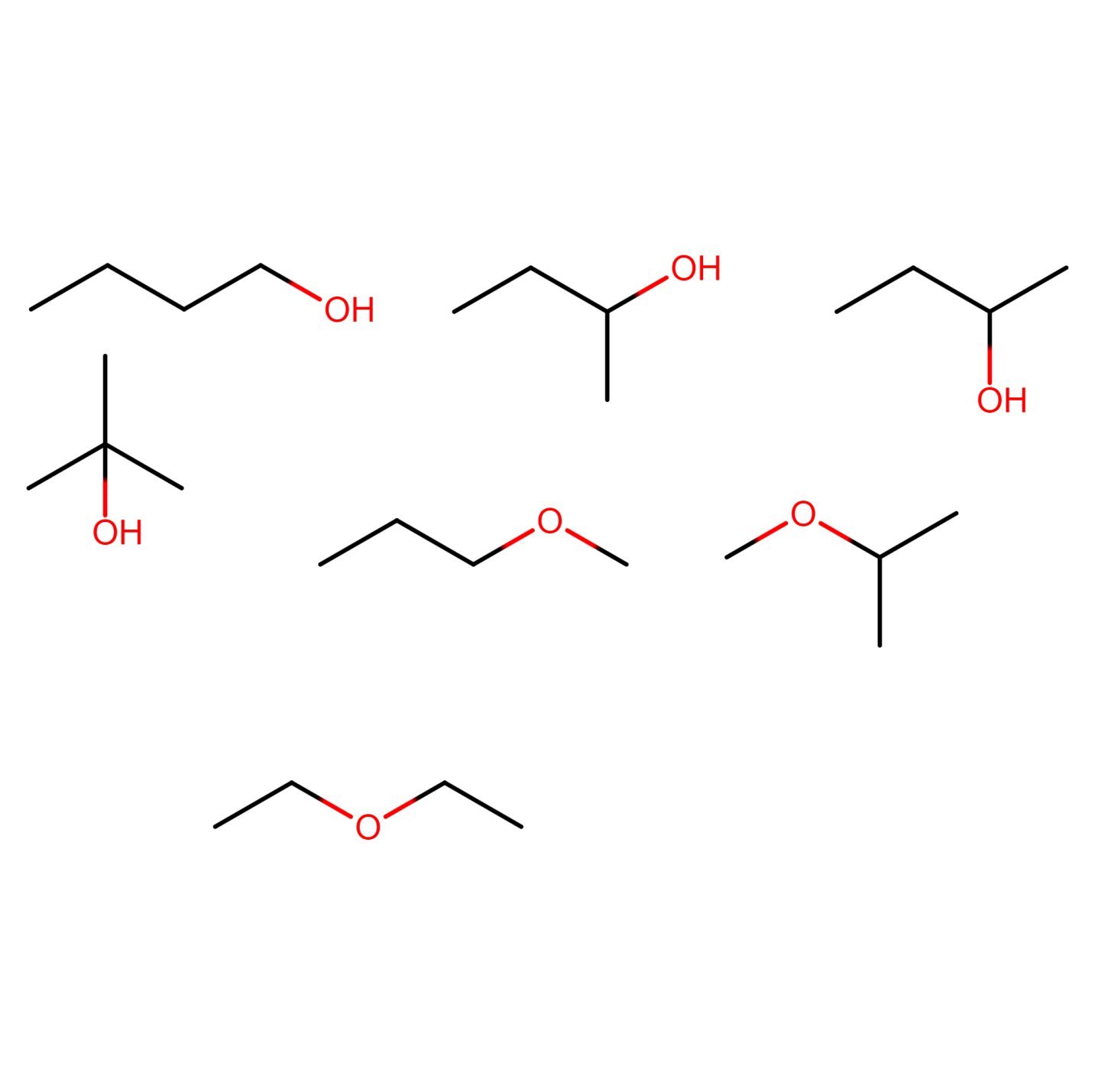
Figure 23) ethoxyethane
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
- NCERT notes Class 11 Chemistry Chapter 13 Hydrocarbons
Isomers of butyne
There are 2 isomers of butyne. 1-butyne structural formula and 2-butyne structural formula is given as:
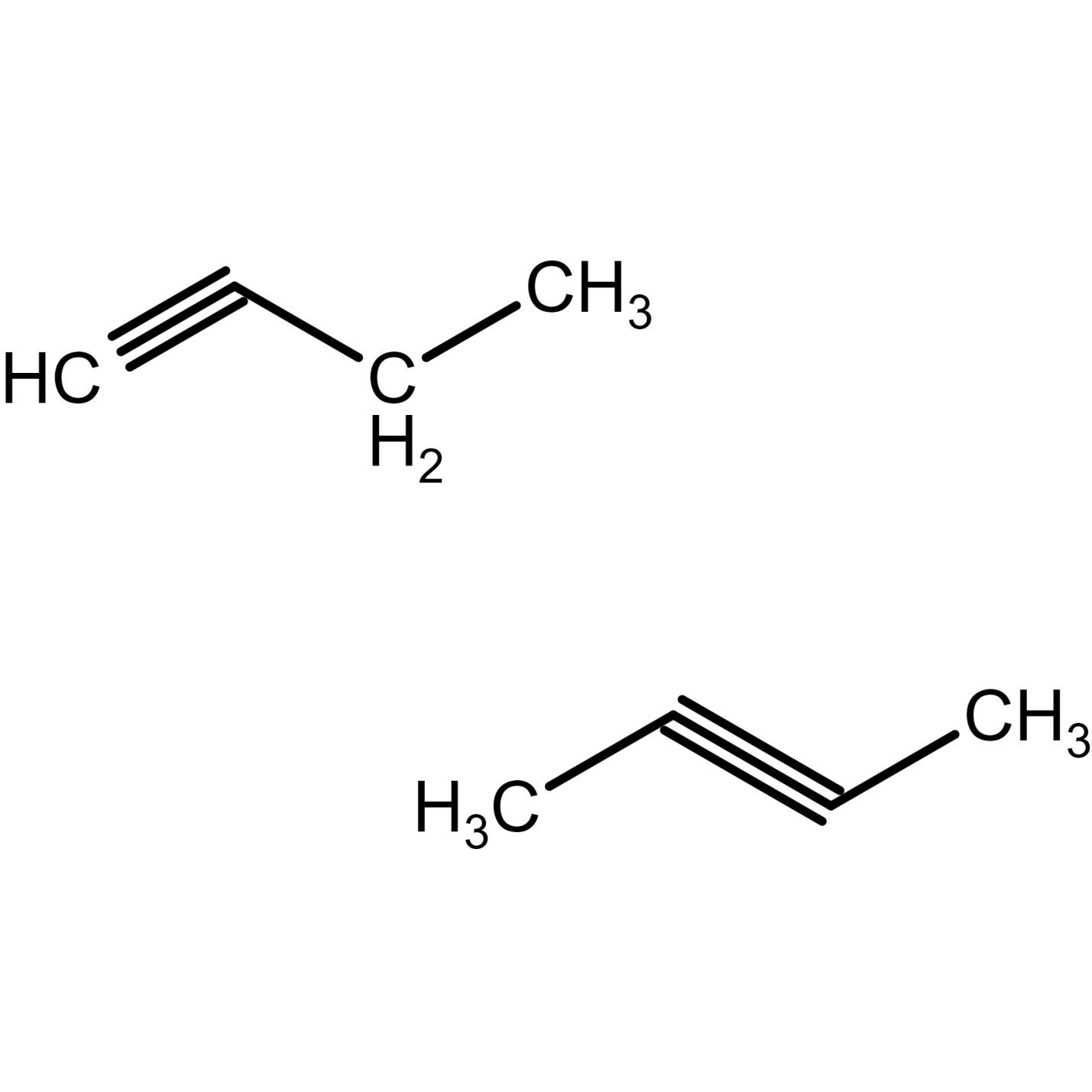
Figure 24) 1-butyne
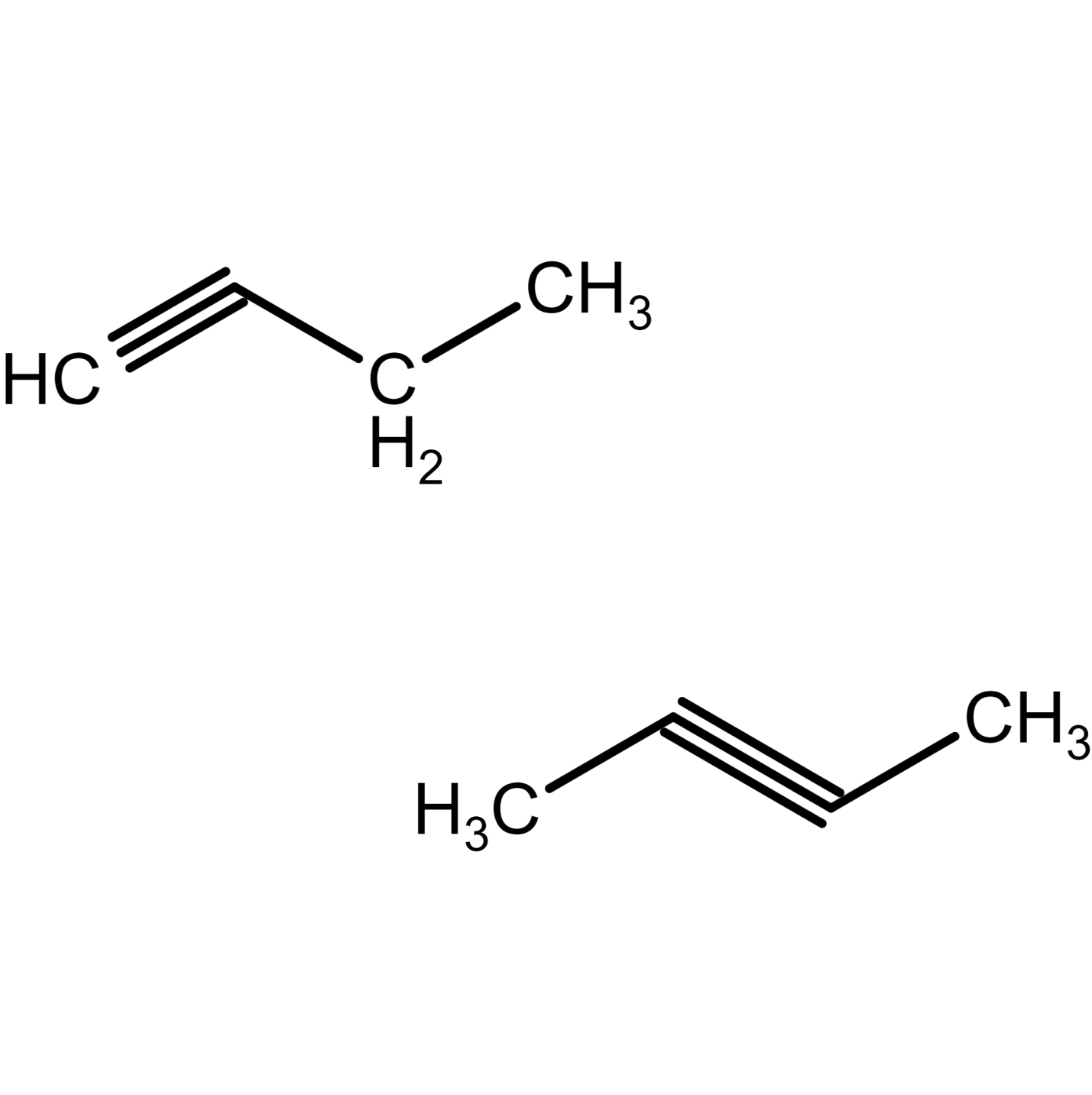
Figure 25) 2-butyne
There are 35 isomers of nonane and 75 isomers of decane.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
D) butane and isobutane
A) The isomer is 2-methylbutane
B) The isomer is n-pentane
C) The isomer is neopentane
N butane and isobutane are chain isomers and show structural isomerism.
Two isomers of butane can exist
Isomers of a substance must have the same molecular formula.
13
isobutane is an isomer of butane.
n-butane and isobutane
2-butyne and 1,3-butadiene are chain isomers.
Methylpropane is an isomer of n butane
Also Read
02 Jul'25 07:16 PM
02 Jul'25 07:16 PM
02 Jul'25 07:16 PM
02 Jul'25 07:15 PM
02 Jul'25 07:15 PM
02 Jul'25 07:14 PM
02 Jul'25 07:14 PM
02 Jul'25 07:14 PM
02 Jul'25 07:14 PM
02 Jul'25 07:13 PM

