Gattermann Koch Reaction Mechanism - Overview, Structure, Properties & Uses, FAQs
What is Gattermann Koch reaction?
The reaction is named after two German chemists Ludwig Gattermann and Julius Arnold Koch. In the Gattermann Koch reaction, instead of using hydrogen cyanide, we use carbon monoxide. The other names of Gattermann Koch reaction are Gattermann Koch formylation reaction and Gattermann salicylaldehyde synthesis.
- What is Gattermann Koch reaction?
- Explain Gattermann Koch Reaction.
- Gattermann Koch Synthesis
- Conclusion of Gattermann Koch reaction Mechanism:
- Electrophilic substitution:
- Duff Reaction Mechanism:
- Vilsmeier Reaction Mechanism:
Also read -
NCERT solutions for Class 11 chemistry and Class 12th chemistry and other subjects can be found from the links given below.
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Explain Gattermann Koch Reaction.
The Gattermann Koch reaction mechanism started with the formation of reactive species and those species are generally acidic in nature. In the Gattermann chemical reaction, the aromatic compounds are formylated. The formylation reaction of the Gattermann Koch reaction took place in the presence of Lewis acid and the catalyst which is being used here is Aluminium chloride. Formylation of the Gattermann reaction occurs with the mixture of hydrogen cyanide and hydrogen chloride. Gattermann Koch formylation is a type of substitution reaction.
The Gattermann Koch reaction is not at all applicable to Phenol and the substrates of Phenol ether. In such a reaction the formyl cation (formyl cation so formed is called protonated carbon monoxide) which is being formed as an intermediate product is highly unstable formyl chloride. Without any further delay, the reaction of such formyl chloride occurs with arene with no initial formation of formyl chloride. It can be noted that in place of Aluminium chloride in the Gattermann Koch reaction as a catalyst Zinc chloride is used as Lewis acid, or instead of carbon monoxide at high temperature is not used but traces of copper chloride or nickel chloride is must work as co-catalyst.
The metal used here is transition metal which serves as a “carrier” that initially reacts with carbon monoxide and produces a carbonyl complex, which is active electrophile after transformation. The Gattermann Koch reaction is being used widely for industrial purposes for the production of benzaldehyde. The main aim of the Gattermann Koch reaction is the attachment of the formal group to the aromatic system.
Gattermann Koch Synthesis
Following are the steps that are included in the Gattermann Koch reaction mechanism or the synthesis of the Gattermann Koch reaction.
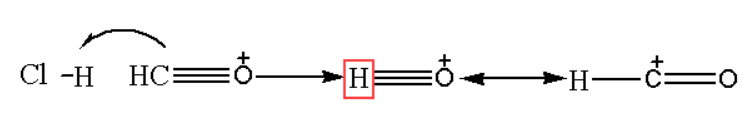
Step 1: Includes the formation of formyl chloride.
In the very first step of the Gattermann Koch reaction, the reactive species are generated as they later in the mechanism react with an aromatic ring. Accepting a proton from hydrochloric acid, carbon monoxide gets protonated which works as a Lewis acid in it. In such a mechanism the molecule changes its resonance structures and becomes positively charged. Showing the reactivity of the hybrid structure formed, carbon here gets a positive charge on it. Further it reacts with aromatic rings, this happens because the species work like electrophile. As we know that in hydrochloric acid the chloride is negatively charged and works as a nucleophile, which behaves as a target of electrophile. The following reaction is shown below:
 +
+
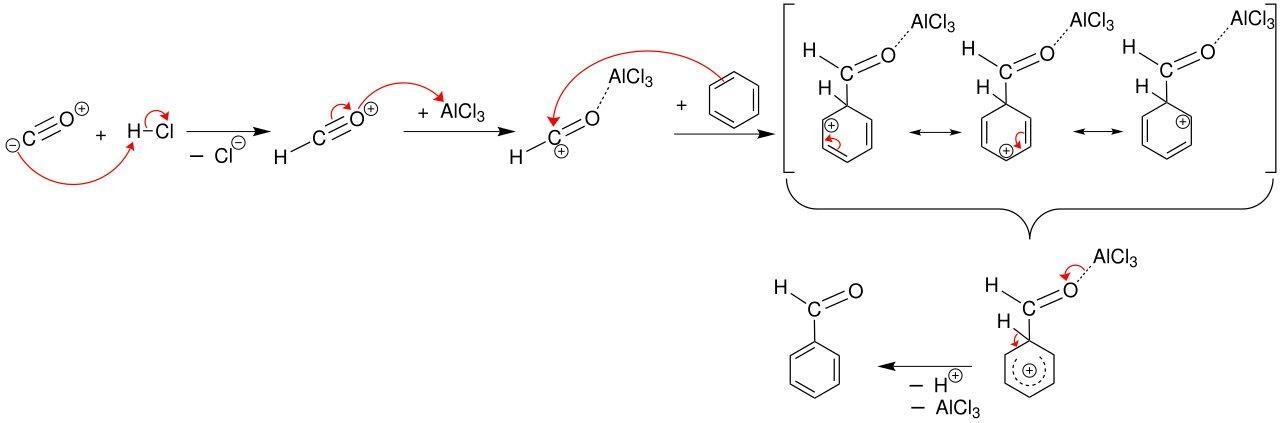
Step 2: Includes the formation of Electrophile.
In the next Gattermann Koch reaction step after getting the intermediate product adding the lewis acid which is aluminium chloride AlCl3to it, works as a catalyst and removes the chloride ion from the species. The obtained species now reverts to formyl cation which is reactive in nature. The reaction can be shown below in compiled form with the final product in the third step.
Step 3: Includes the formation of Benzaldehyde.
Gattermann Koch reaction is substitution reaction so here electrophilic aromatic substitution can be seen at the aromatic ring. The aromatic ring here behaves as a nucleophile which donates an electron pair to the intermediate formyl cation. The loss of aromaticity will no longer be there in the reaction and can be resolved by the expulsion of a proton. Such type of mechanism is called the Gatterman Koch formylation. The complete Gattermann Koch reaction is shown below where we get the final product.
Conclusion of Gattermann Koch reaction Mechanism:
In the last step, we see that the formyl group is attached to an aromatic ring which is done with the help of the Gattermann Koch reaction. The above example of the mechanism shows that benzaldehyde can be prepared from the treatment of benzene with carbon monoxide and of course the action of hydrochloride on it, the process occurs in the presence of a catalyst which is aluminium chloride. One of the major conclusion that can be drawn from the reaction is that the Gattermann reaction is different from the Gattermann Koch reaction.
The Gattermann reaction is named after the scientist Ludwig Gattermann whereas the Gattermann Koch reaction is named after two scientists from Germany, Ludwig Gattermann and Julius Koch In the Gattermann reaction reaction follows the substitution reaction of organic compounds in which we can formylate the aromatic compounds. In the Gattermann reaction conditions are different as in the involvement of hydrogen cyanide and hydrochloric acid can be seen here, on the other hand in the Gattermann Koch reaction, instead of using hydrogen cyanide carbon monoxide is being used. Gattermann reaction can be applied to phenol and substrates of phenol ether which can be applied in the Gattermann Koch reaction.
Example: In the presence of anhydrous aluminium chloride AlCl3 ,what are the products formed when benzene reacts with CO and HCl.
In the Gattermann – Koch reaction: In an acidic medium in presence of anhydrous aluminum chloride AlCl3, benzene is treated with carbon monoxide and produces the product benzaldehyde. Here aluminum chloride works as a catalyst, which is Lewis acid. The Gattermann Koch reaction is an electrophilic substitution reaction.
Related Topics link, |
Electrophilic substitution:
Electrophilic substitution can be seen in benzene because benzene is electron rich and undergoes such a reaction. Complete step-by-step substitution reaction can be seen here.
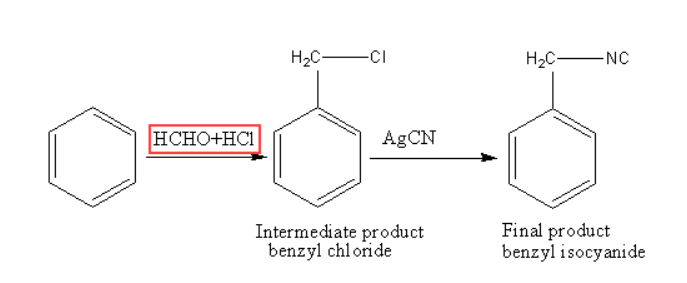
The reagents used in such reactions are formaldehyde and hydrochloric acid and benzene work as substrate. This type of reaction is the so-called Chloromethylation reaction of benzene. In the presence of hydrochloric acid, the benzene reacts with formaldehyde to give the product as benzene methyl chloride. This reaction is called the Electrophilic substitution reaction. The reaction may include a step wherein the first step we see the intermediated product formed is an electrophile.
Step 1: In this step, the reaction between benzene and HCHO, HCl takes place to give the species positive methyl chloride, which is an electrophile.
Step 2: Further this electrophile reacts with benzene to produce benzyl chloride, after this product reacts with AgCN to produce the final product which is benzyl isocyanide. AgCN is covalent in which C and N can both donate the electrons but reaction (attack) takes place on N as donating the electron pair gives rise to the major product formed in this reaction which is benzyl isocyanide. The reaction mechanism is shown below.
Duff Reaction Mechanism:
The Duff reaction was named after the scientist Cooper Duff. The reaction can also be called hexamine aromatic formylation which is used in organic chemistry for the mechanism procedure related to benzaldehydes. Duff reaction takes place when benzaldehyde reacts with hexamine which is the formyl carbon source. In Duff’s reaction, the electrophilic species here is the iminium ion, which is the initial product in this reaction which further hydrolyzes to the aldehyde.
Initially, we get the oxidation state of benzylamine, in addition to the aromatic ring. After this, the oxidation state of aldehyde is achieved when an intramolecular redox reaction raises the benzylic carbon. And in the final step we see that the acid hydrolysis occurs by water which gives the oxygen in the final stage of the reaction mechanism of Duff reaction.
Below are the reaction mechanism showing you the exact synthesis process that occurs in the Duff reaction:
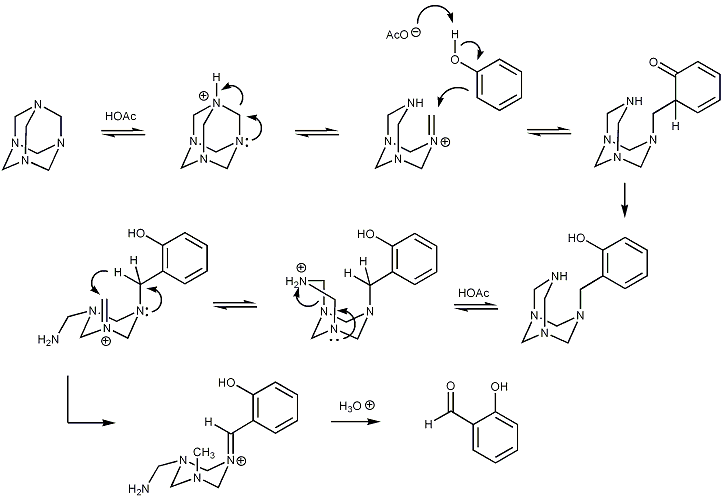
Also Read:
Below are the links for Solution to the Class 12 chemistry problems which can be assess in case a students finds difficulty to solve the questions.
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Vilsmeier Reaction Mechanism:
Vilsmeier reaction is the alternative reaction mechanism of Friedel-craft reaction, which helps in avoiding the use of strong Lewis acid, that is Aluminium chloride. Vilsmeirer reaction mechanism is very specifically used for formylation. The reaction of Vilsmeier can be completed with the help of three major steps. In the first step, the formation of iminium cation occurs which is the intermediate product, which further tends to create the second step in which nucleophilic substitution occurs. And in the last final step hydrolysis of an iminium salt occur which gives us the major product. The reaction mechanism is shown below:
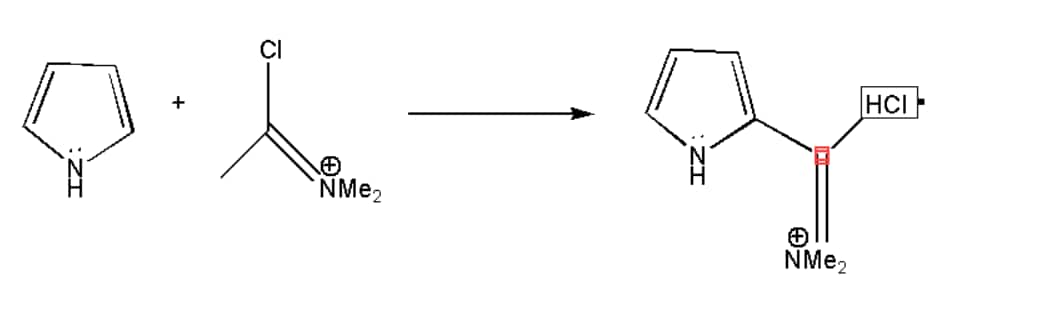
Example:
What do you mean by the pyrrole ring?
It is defined as a ring structure consisting of four carbon atoms and one nitrogen atom of organic compounds of heterocyclic series is so-called a Pyrrole ring. Talking about the simplest member of the pyrrole family, pyrrole itself is considered as the simplest one, with molecular formula C4H5N.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The Gattermann reaction or the Gattermann formylation is an organic chemical reaction in which aromatic compounds are formylated with the help of a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a catalyst. The Gattermann reaction is also called Gattermann salicylaldehyde synthesis, the catalyst used in the reaction is Lewis acid i.e.aluminium chloride.
A chloro-iminium salt is found as a weak electrophile which works great in this reaction. Because of chloro-iminium salt, the Vilsmeier–Haack reaction works better with electron-rich carbocycles and also heterocycles.
The reaction between hexamethylenetetramine and boric acid in the presence of glycerol, and phenol is heated salicylaldehyde is formed. This reaction is termed a Duff reaction.
Delocalized electrons are present in the planar molecular geometry of benzene above and below the plane of the ring. Therefore, it is an electron-rich species. Due to this, it is more attractive to electron-deficient species. So we can say that yes, it shows electrophilic substitution reactions very easily.
Also Read
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:12 PM


