Gabriel Phthalimide Synthesis, Mechanism - Reaction with FAQs
Gabriel phthalimide synthesis
The Gabriel synthesis or Gabriel phthalimide synthesis is named after the German chemist Siegmund Gabriel. Gabriel phthalimide synthesis is a reaction that involves conversion of primary alkyl halides into primary amines using alkyl halides. Conventionally, the Gabriel synthesis uses potassium phthalimide.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Gabriel phthalimide synthesis
- Gabriel phthalimide reaction
- Gabriel synthesis mechanism
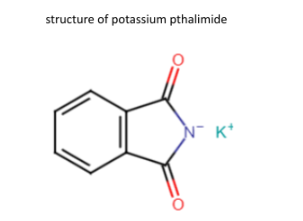
The Gabriel phthalimide synthesis or Gabriel synthesis has applications in the alkylation of sulfonamides and imides, followed by their deprotection, to obtain amines. The alkylation of ammonia is frequently an extensive and inefficient route to amines. In the Gabriel phthalimide synthesis, phthalimide anion is recruited as a proxy of H2N−.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Gabriel phthalimide reaction
The main objective of Gabriel phthalimide synthesis is to form primary amine (RNH2). Gabriel synthesis involves reaction of potassium hydroxide with the phthalimide which forms a good nucleophile in the form of an imide ion. The imide ion attacks alkyl halide via nucleophilic substitution reaction and leads to the formation of an intermediate named as N-alkyl phthalimide. Phthalimide then undergoes hydrolysis which yields a primary alkyl amine. However, aryl amines cannot be prepared through Gabriel synthesis because aryl halides do not undergo simple nucleophilic substitution. Gabriel synthesis has an advantage of eluding the possibility of over alkylation.
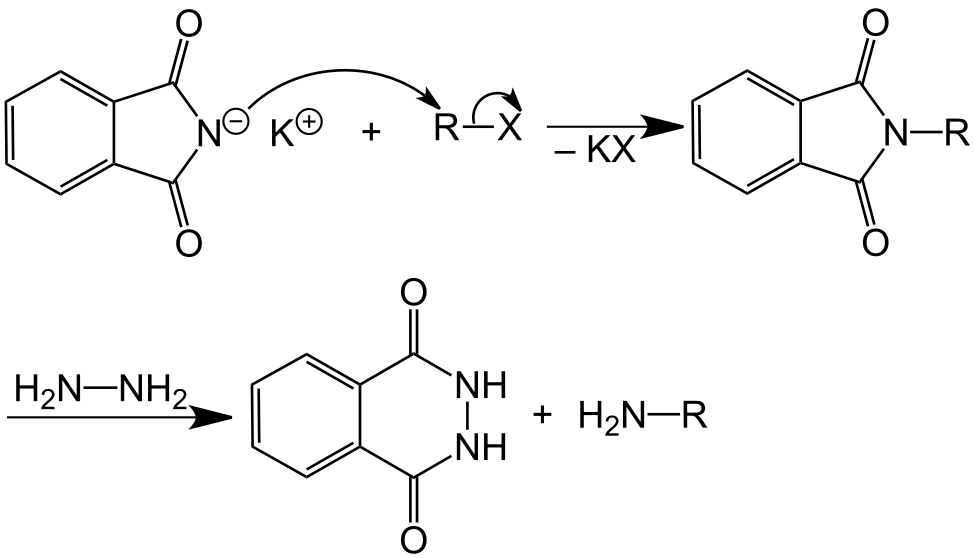
Before discussing the mechanism, we could write the overall Gabriel phthalimide reaction as:
Gabriel synthesis mechanism
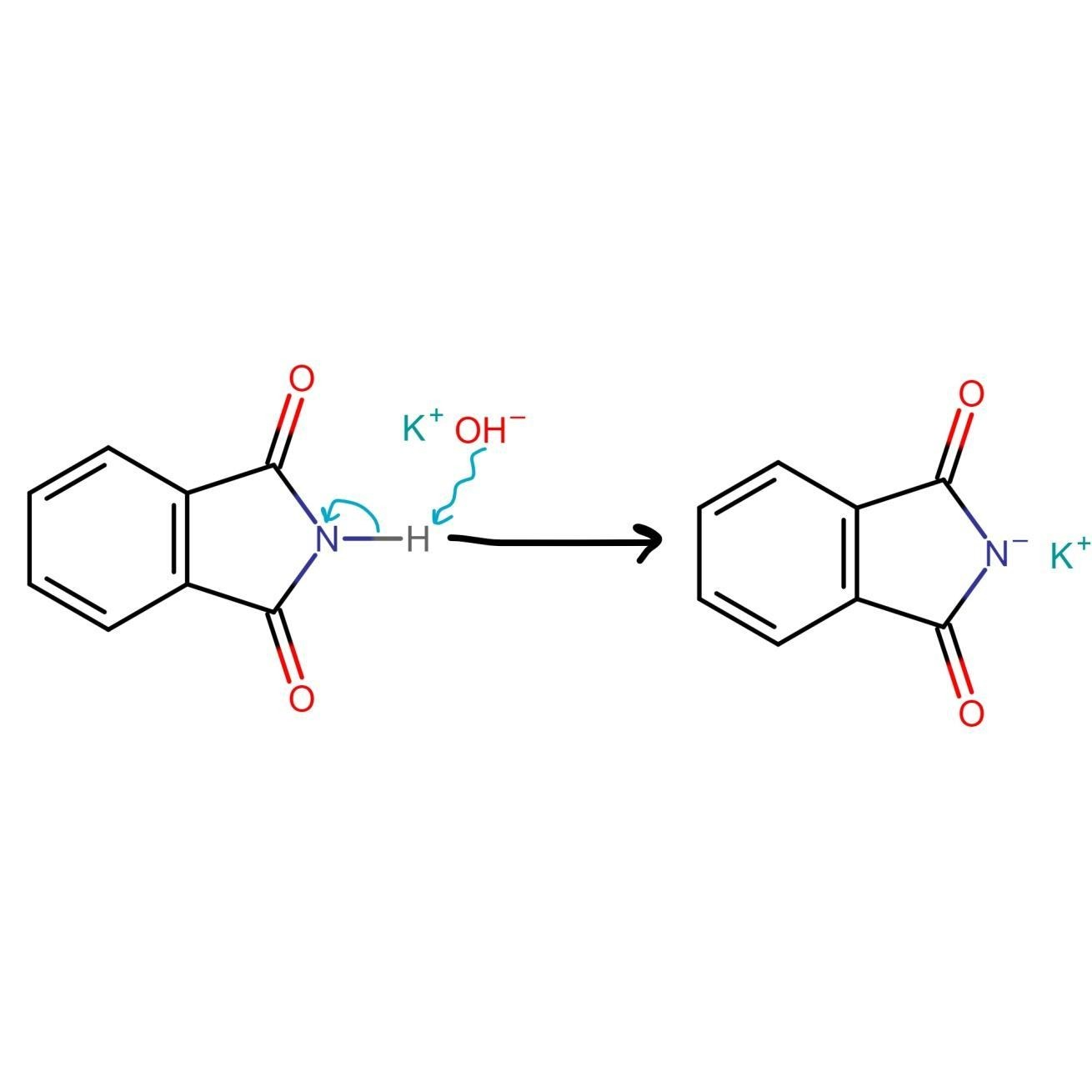
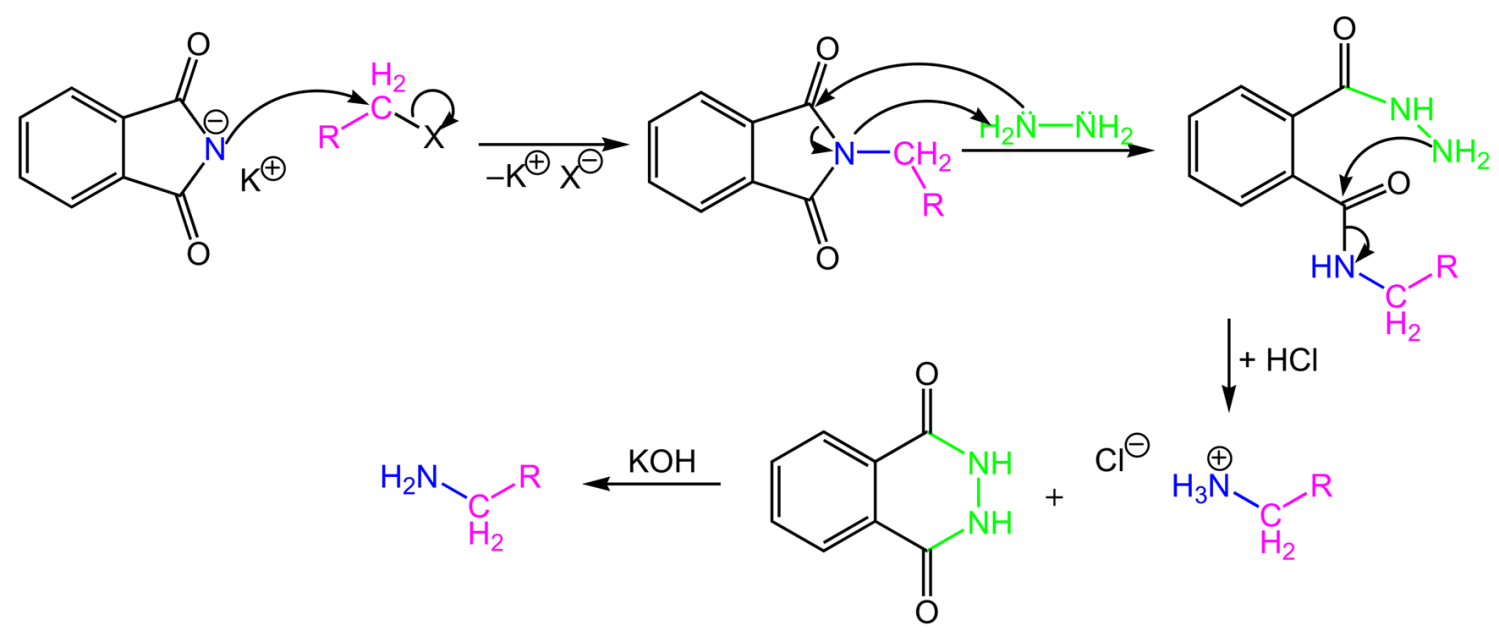
As we discussed above, Gabriel phthalimide reaction is used for the preparation of primary amines and Gabriel phthalimide reaction is a nucleophilic substitution reaction. Now, we need to understand how this reaction proceeds and how different groups interact with each other to produce primary amines as endgame. The reaction initiates with conversion of phthalimide into potassium phthalimide by addition of alkali base KOH or NaOH. Removal of protons from the nitrogen of phthalimide takes place; this is called deprotonation of nitrogen. In some books this step is skipped.
Gabriel phthalimide synthesis then proceeds with potassium phthalimide. The nitrogen of potassium phthalimide gains a negative charge due to deprotonation. This negatively charged nitrogen can now act as a nucleophile and react with alkyl halide via bimolecular nucleophilic substitution reaction or SN2 reaction.
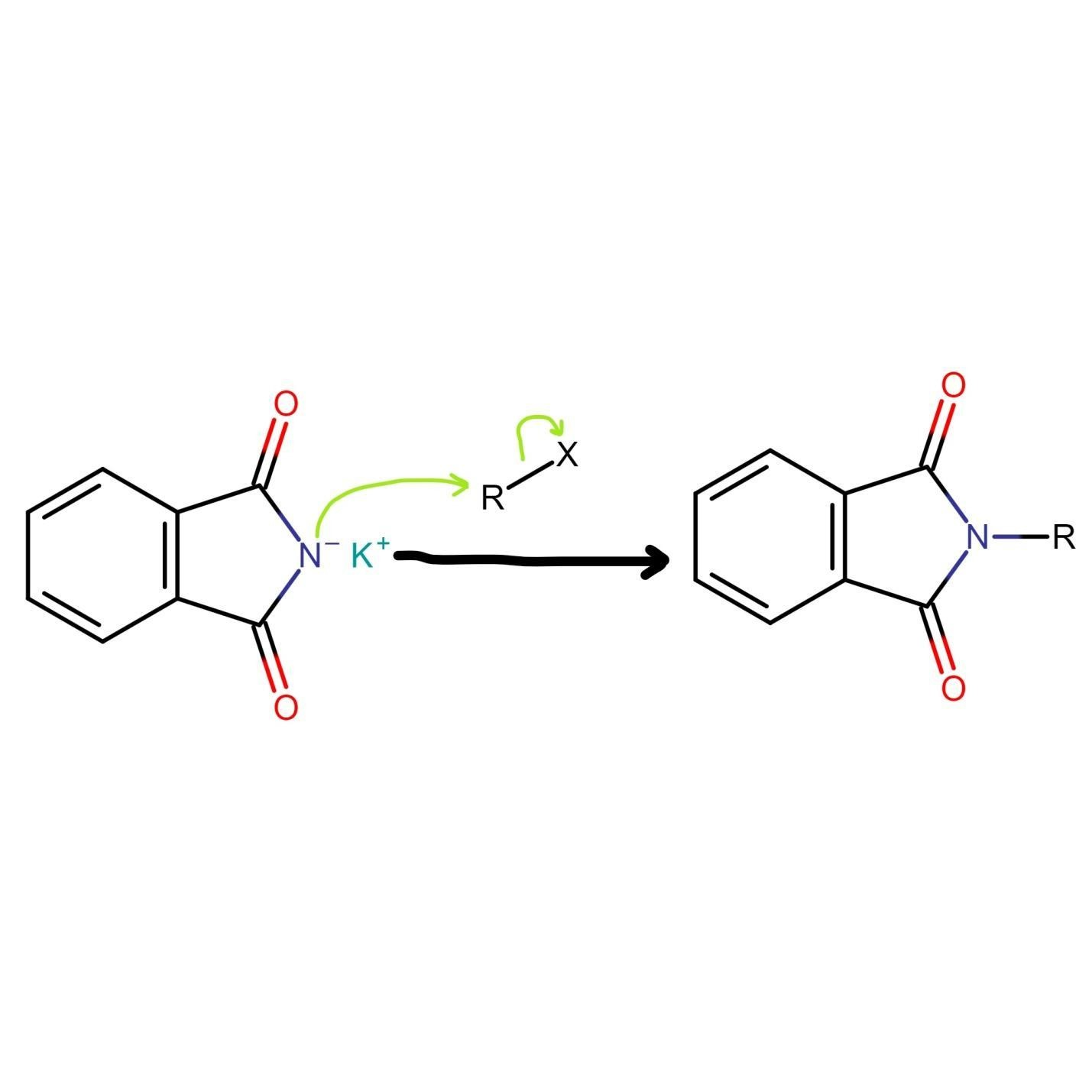
The nucleophilic nitrogen of Gabriel phthalimide attacks the electrophilic carbon of alkyl halide. The potassium ion from KOH combines with halogen of alkyl halide and forms KX. The alkyl group of alkyl halide attaches to nucleophilic nitrogen which results in the formation of N-alkyl phthalimide (structure given below).
| Related topics link, |
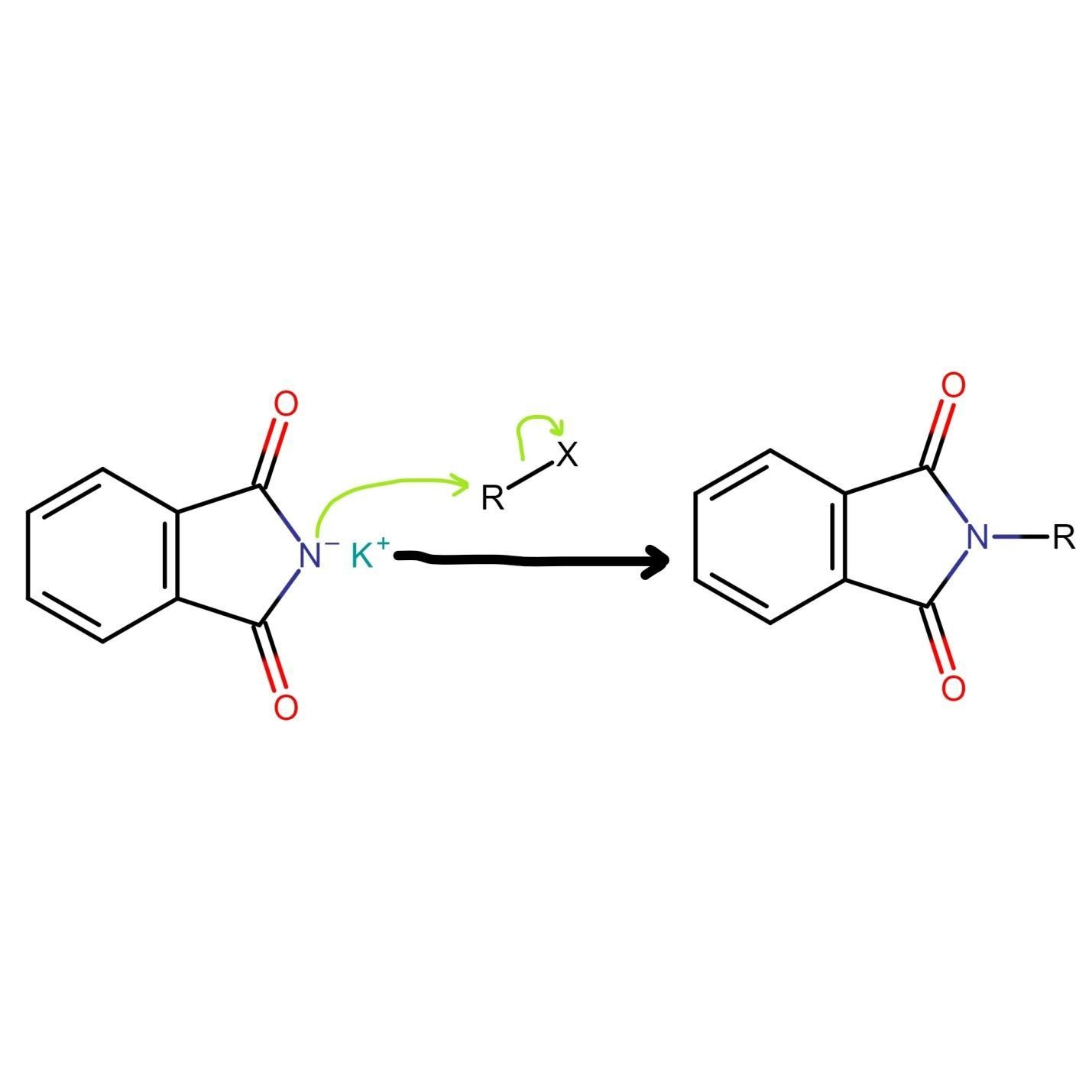
Figure.1. Structure of N-alkyl phthalimide
At this stage of Gabriel phthalimide reaction, there are a couple of variations for generating amines. Generally, hydrazine (NH2NH2) is used via the Ing–Manske procedure which produces a precipitate of phthalhydrazide along with the primary amine. Processes like acidic hydrolysis or basic hydrolysis can sometimes be done. Gabriel synthesis involving acidic hydrolysis liberates amine salt from the primary amine. We will discuss how reaction proceeds with addition of hydrazine. Hydrazine is a nucleophile which on adding to carbonyl carbon gives nucleophilic acyl substitution with N-alkyl phthalimide.
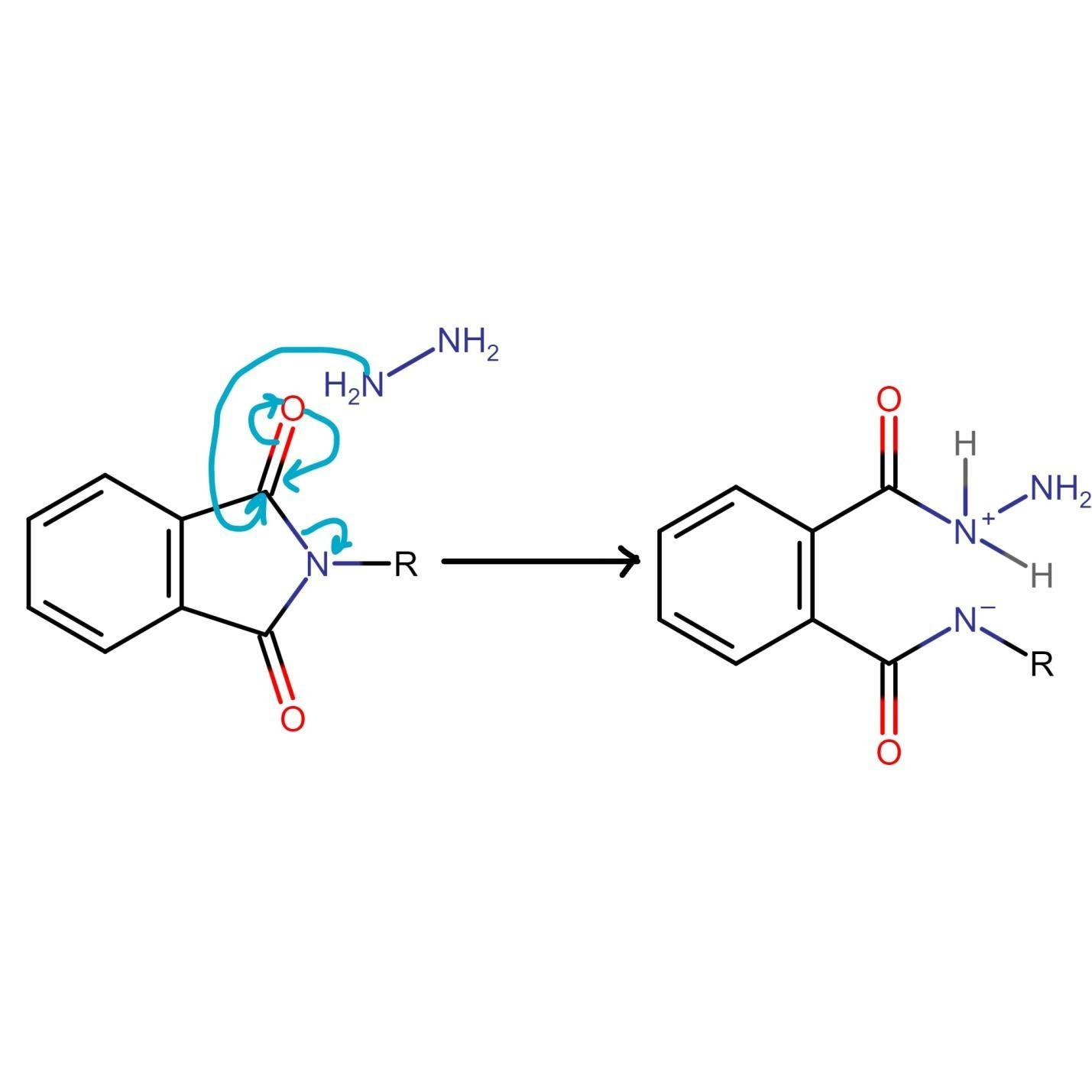
The nitrogen of phthalimide then participates in deprotonation of the protonated hydrazine.
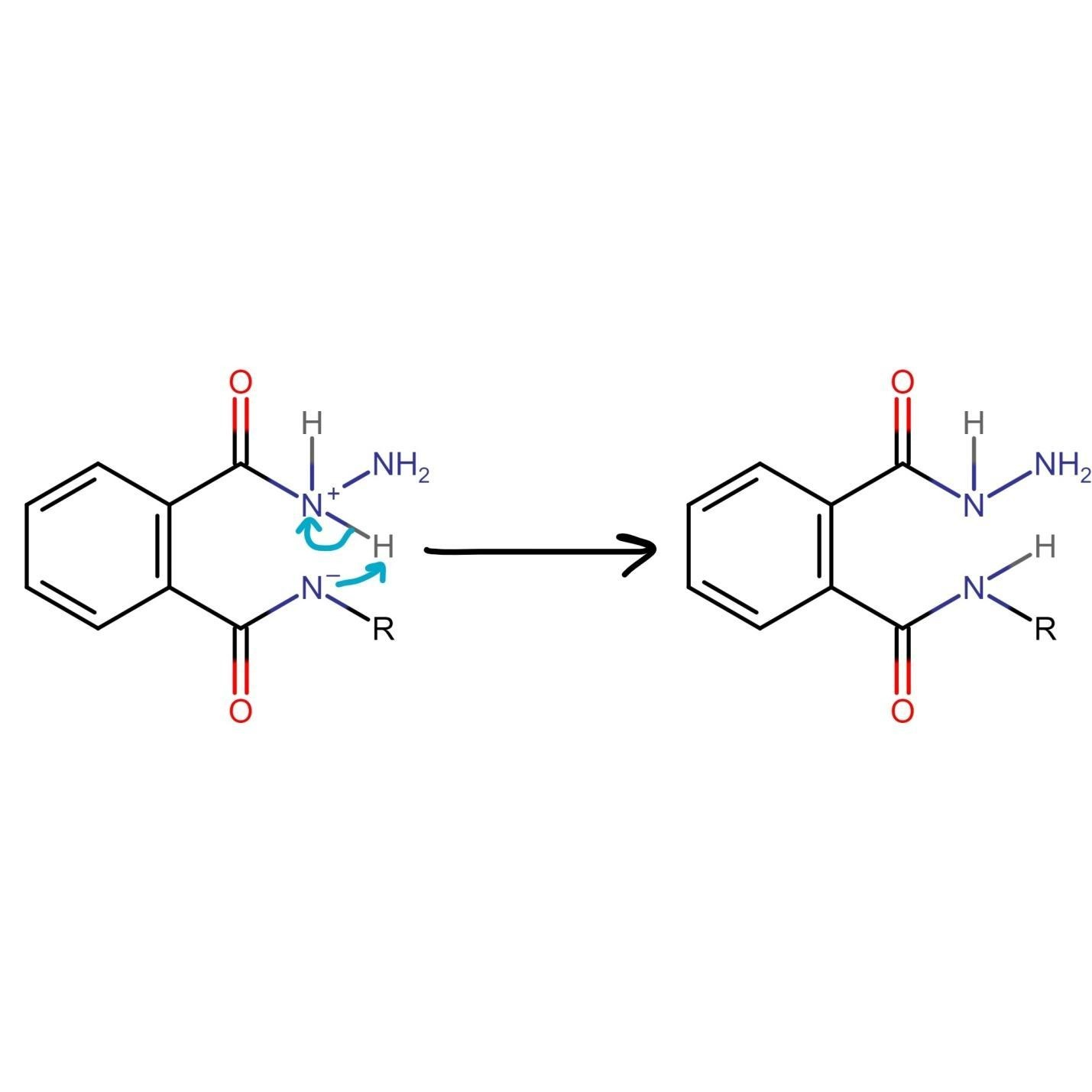
The nitrogen of hydrazine is deprotonated by nitrogen of phthalimide which leads to the formation of an uncharged species.
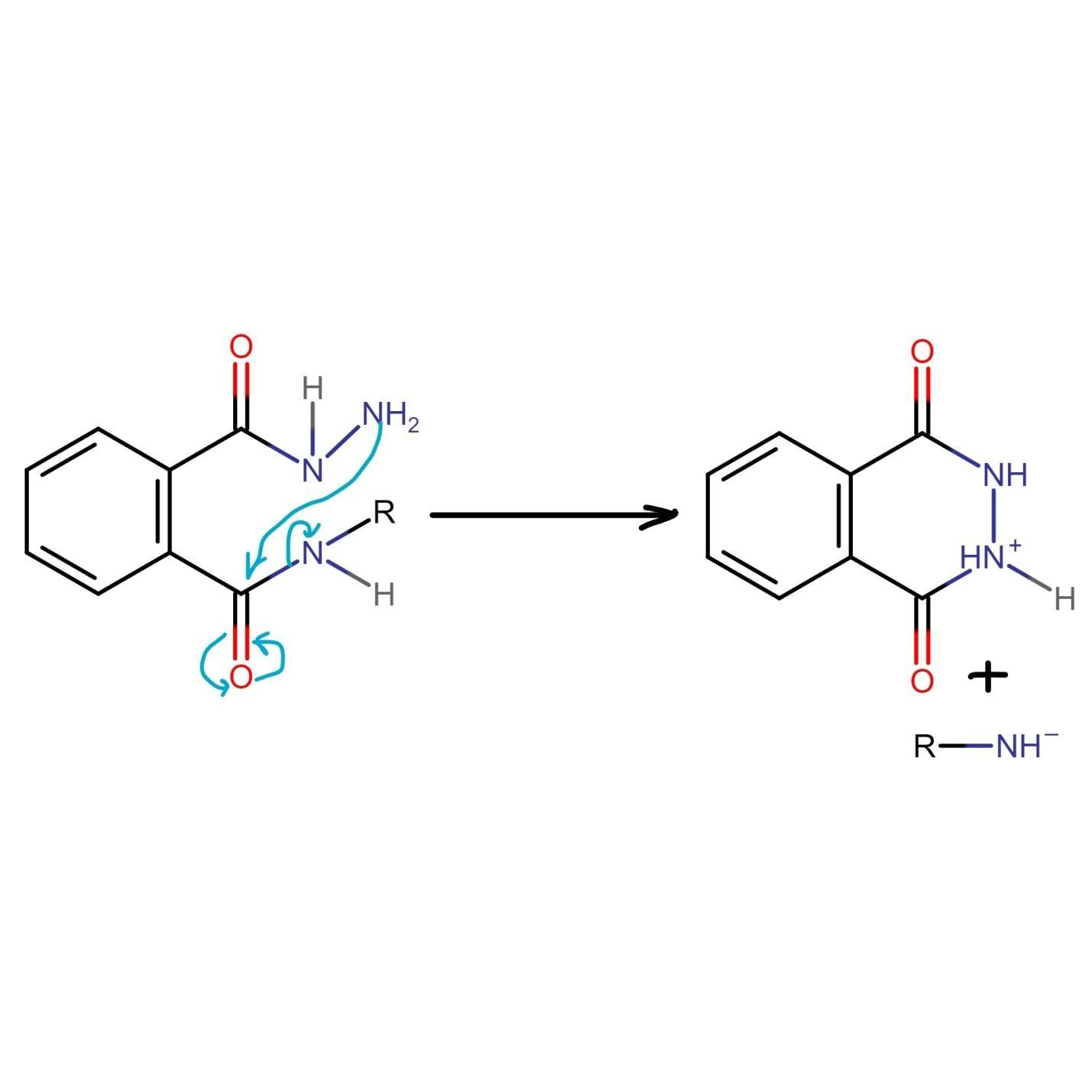
The unreacted NH2 group of hydrazine undergoes nucleophilic acyl substitution and attacks the carbonyl group liberating amine. Both species generated in this step of Gabriel phthalimide synthesis are charged which need to be taken care of.
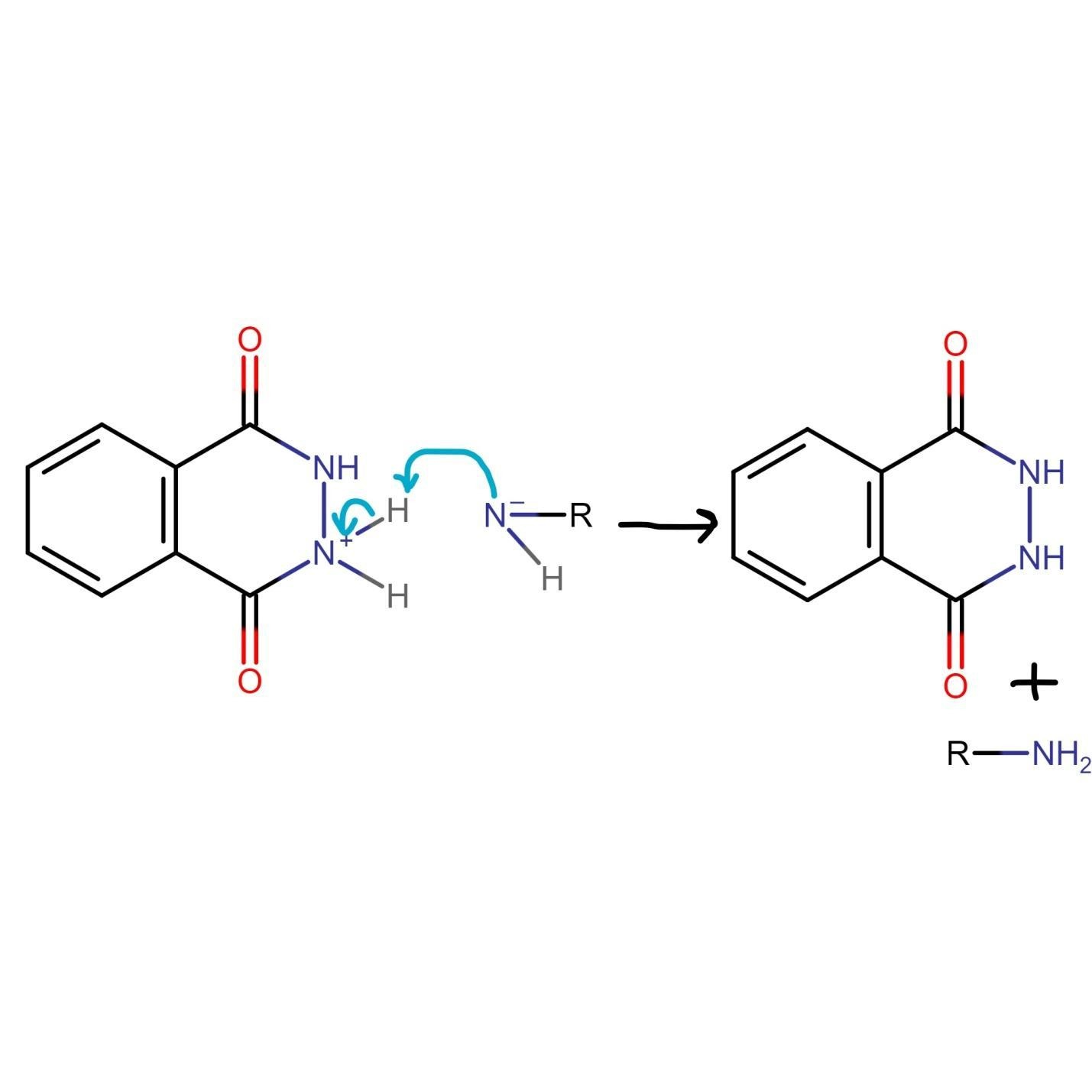
The final step of Gabriel phthalimide reaction involves neutralization of charge which results in formation of primary amine RNH2. This step marks the end of Gabriel phthalimide synthesis.
Alternate methods of Gabriel synthesis
Gabriel phthalimide synthesis using hydrazine often produces low yields or side products. Consecutively, it is not easy to separate phthalhydrazide. This is the main problem which leads to the formulation of other methods to liberate amines from phthalimide. Alternative reagents like the sodium salt of saccharin and di-tert-butyl-iminodicarboxylate are used in place of hydrazine.
Such reagents are electronically similar to phthalimide salts and consist of imido nucleophile. Since Gabriel synthesis is ineffective for secondary alkyl halides, these reagents ensure reactivity of secondary alkyl halides too. These reagents hydrolyze more readily and allow the production of secondary amines. Another common alternative includes acid hydrolysis and base hydrolysis of phthalimide.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Gabriel phthalimide synthesis involving acid hydrolysis
Gabriel synthesis initiates with H3O+ ion which is made by addition of any acid in aqueous solution. The hydronium ion protonates one of carbon of the carbonyl groups of phthalimide and addition of water takes place which in turn leads to the cleaving of N-alkyl phthalimide. Another molecule of nucleophilic water then attacks the other carbon of carbonyl carbon which develops charge on the molecules. This extra charge forces RNH2 to detach from carbonyl carbons. The end of Gabriel synthesis is marked by substitution of RNH2 by OH from the N-alkyl phthalimide which results in formation of an amine.
Gabriel phthalimide synthesis involving basic hydrolysis
Gabriel phthalimide reaction initiates with an attack of OH- on one of the carbonyl carbon. Nucleophilic OH ion attacks carbon of carbonyl group by nucleophilic substitution reaction. This results in cleaving of N-alkyl phthalimide. During this process the oxygen atom of the carbonyl group gains a negative charge. Another molecule of OH ion attacks the second carbonyl group. This process of charge transfer between nucleophile and electrophile to gain stability and neutralize molecules forces RNH2 to detach from carbonyl carbon. The nitrogen is replaced by O- ions. This marks the end of nucleophilic substitution reaction and hence end of Gabriel phthalimide synthesis.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Gabriel phthalimide synthesis is used for preparation of primary amines
Gabriel synthesis is used for the preparation of primary amines.
The Gabriel synthesis or Gabriel phthalimide synthesis is named after the German chemist Siegmund Gabriel. Gabriel phthalimide synthesis is a chemical reaction that converts primary alkyl halides into primary amines using alkyl halides. Conventionally, the Gabriel synthesis uses potassium phthalimide
Named after | Sigmund Gabriel |
Reaction type | Substitution reaction |
The main objective of Gabriel phthalimide synthesis is to form primary amine (RNH2). Gabriel synthesis involves reaction of potassium hydroxide with the phthalimide which forms an imide ion which is a good nucleophile. Nucleophilic substitution takes place from the imide ion on the alkyl halide. This leads to the formation of an intermediate named as N-alkyl phthalimide. Phthalimide then undergoes hydrolysis which yields a primary alkyl amine. However, aryl halides do not undergo simple nucleophilic substitution and thus aryl amines cannot be prepared through Gabriel synthesis. Gabriel synthesis has an advantage of eluding the possibility of over alkylation.
The method by which aniline cannot be prepared is Gabriel phthalimide synthesis. – Aniline is produced by degradation of benzamide with bromine in alkaline solution. This reaction is called Hoffmann bromamide degradation.
N ethyl phthalimide on hydrolysis gives ethyl amine. N-alkyl phthalimide on hydrolysis gives primary amines via nucleophilic substitution reaction mechanism.
Methylamine can be prepared by Hoffmann bromamide reaction which involves reaction of ammonia with methanol in the presence of an aluminosilicate catalyst.
Also Read
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:12 PM


