How Many Electrons is Equal to 1 Coulomb
Introduction
Electrons are the subatomic particles constituting the flow of current in a wire. The charge on a single electron is denoted by ‘e’. Coulomb is the unit of Electric charge in the SI Unit System. The quantity of charge on an object can be calculated by multiplying the elementary charge and the number of elementary charges on the object.
What are the electrons?
The electrons are the subatomic particles having a negative charge on them. Electrons have an electric charge which is equal to
\begin{equation}
1 \mathrm{e}=1.602 \times 10^{-18} \mathrm{C}
\end{equation}
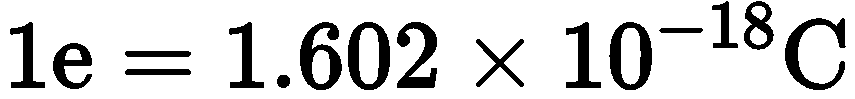
An electron constitutes the electric current which flows through a wire.
The mass of an electron at rest is equal to 9.11 \times 10^{-31}
![]() kilograms(kg).
kilograms(kg).
What is a coulomb?
Coulomb is the amount of electric charge delivered by a one-ampere constant current through a wire in one second. Coulomb is represented by the symbol ‘C’.
1 coulomb is equal to how many electrons?
As we know charge on
\begin{equation}
1 \mathrm{e}=1.602 \times 10^{-18} \mathrm{C}
\end{equation}
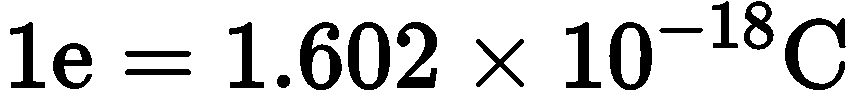
And the total given charge = 1C
Total no of electrons =

\begin{equation}
\frac{1}{1.602 \times 10^{-19}}=6.24 \times 10^{18} \mathrm{e}^{-}
\end{equation}
So the 1 coulomb has 6.24 \times 10^{18} e.
![]()
Solved Questions:
Ques 1: How many electrons are there in 4 coulombs of charge?
Ans: Charge on
\begin{equation}
1 \mathrm{e}=1.602 \times 10^{-18} \mathrm{C}
\end{equation}
![]()
total given charge = 4C
Total no of electrons =
\begin{equation}
4 \div 1.602 \times 10^{-19} \mathrm{C}=2.496 \times 10^{19} \mathrm{C}
\end{equation}
![]()
Ques 2: How many electrons are there in 10 coulombs of charge?
Ans: charge on
\begin{equation}
1 \mathrm{e}=1.602 \times 10^{-18} \mathrm{C}
\end{equation}
![]()
total given charge = 10C
Total no of electrons =
\begin{equation}
10 \div 1.602 \times 10^{-19} \mathrm{C}=6.24 \times 10^{19} \mathrm{C}
\end{equation}
![]()
Frequently Asked Questions (FAQs)
An electroscope is used to detect and measure the electric charge on an object.
Electrons in a wire flow from the Negative terminal to Positive Terminal
To calculate the charge on an object, we use
Q= ne,
Where n= number of electrons given
e= charge on the elementary electron.
1 C is bigger than the charge on an electron. As we know 1 coulomb has 6.24 \times 10^{18} e.
![]()
As per the international system of units,Coulomb is a unit of electric charge.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
