Zwitterion - Definition, Example, Structure, FAQs
Zwitterion is basically derived from the German word Zwitter, which means hermaphrodite or roughly we can say hybrid ion. A zwitterion consists of two main functional groups. Zwitterion generally contains both the charges, in other words we can say that zwitterion is electrically neutral. The net formal charge ion zwitterion is zero. In case of dipolar compounds zwitterion are not designated as dipolar compounds but these are inner salts. This can be distinguished with the help of plus and minus sign on amine oxide, which signifies the formal charge.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is zwitterion or Zwitterion Definition:
- Zwitterion Example:
- Zwitterion structure of alanine:
- Zwitterion structure of glycine:
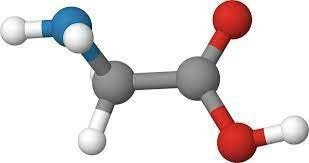
What is zwitterion or Zwitterion Definition:
Zwitterion can be defined as the molecule which contains both negatively charged ions as well as positively charged ions. Amino acids can exist in dipolar forming the solid state and are termed as Zwitterion. For a compound to be zwitterion it is important to understand the concept of pH. As it is important to specify the cation and anion as anion can be formed when a sufficient amount of alkaline solution is available whereas cation can be formed when sufficient amount of acid is available. The polymers of zwitterion are also available in the form of positive and negative functional groups which are present at the end of the chain. The nature of zwitterion is amphoteric in nature. Electrical neutrality of the zwitterion does not migrate during electrolysis (neither anode or cathode).
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
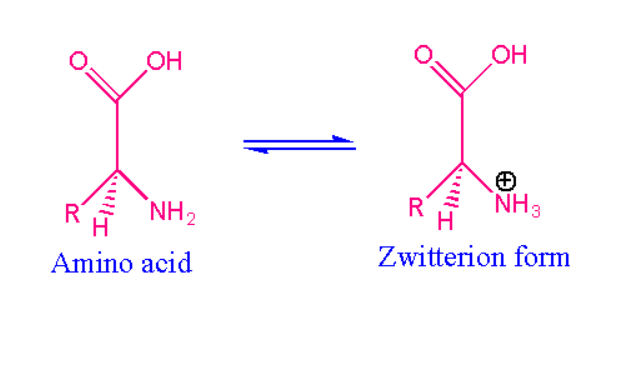
Zwitterion Example:
The best example of zwitterion is alpha amino acids. Due to the formation of zwitterion alpha amino acids show the crystalline properties. While dissolving alpha amino acids in aqueous solution it gives zwitterion. For example, when we dissolve R-CH- NH3-COOH in aqueous medium then oxygen atom present in the functional group pulls electron from the hydrogen. Here hydrogen gets positive charge and oxygen becomes negative, Now the hydrogen ion gets bonded with the nitrogen atom. Therefore, nitrogen gains the positive charge thereby forming the NH3.
The other examples of zwitterion are as follows: trimethylglycine, psilocybin, cocamidopropyl. The amino acids which is optically inactive are termed as glycine.
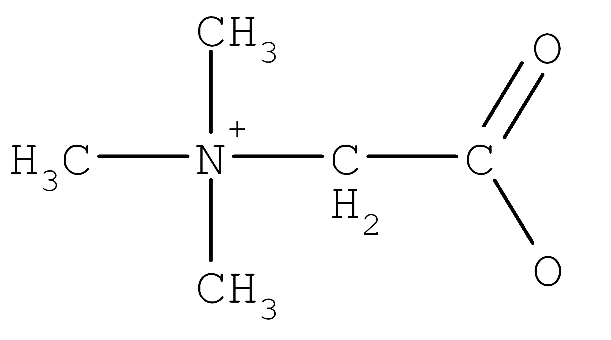
Betaine is also one such example of zwitterion which is a chemically neutral compound. Betaine is a special type of zwitterion as it does not have any hydrogen atom in it. It contains positively charged ammonium or phosphonium groups and negatively charged carboxylate groups.
Sulfamic acid exists as a neutral as well as zwitterion form.
Zwitterionic forms:
The compounds of zwitterion are formed like ampholytes which contain both acids and bases in their molecules.
In zwitterion the charged atoms are held together by bond i.e. covalent bond.
Zwitterion form of amino acids:
As we discussed earlier, the best examples of zwitterion are amino acids. As amino acids contain amino group and carboxyl functional group i.e. individually amino acids are not zwitterion it can behave only in aqueous solution by attaining the proton on hydrogen atom and loses to become carboxyl group with negative charge. Such a reaction is called an isomerisation reaction. At neutral pH range of water.
The equilibrium exists between parent amino acid and its zwitterion. Amine is present in the amino acid group which is the stronger basic group while carboxylic is an acidic group. Zwitterion of an amino exists with pH range equal to isoelectric point. Based on the properties of amino acids each has its own pH value. Above and below the isoelectric point the molecule shows the pH values and has net charge which directly depends on the pH of the molecule and pH of the solution.
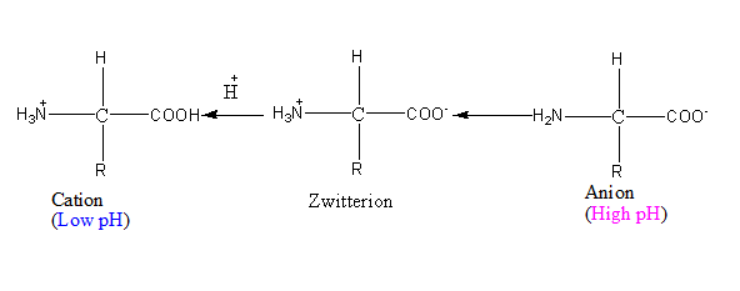
Isoelectric point:
Isoelectric points can be defined according to specific pH range. The net electric charge is found to have zero value at characteristic pH value. Such a point is defined as an isoelectric point.
Isoelectric point of amino acids:
The solubility of the molecule is affected by its isoelectric point value at a certain pH range.
Checking the pH as according to isoelectric point:
Related Topics Link, |
When pH is less than isoelectric point.
The presence of hydrogen ions in the solution is found to be more when the value of pH is less than isoelectric point. This causes protonation as the excess hydrogen ions attract the carboxylate ion. Leaving only positive charge on amine, as carbohydrate ions are protonated making the compound neutral. In the overall scenario amino acids have charge +1. Below showing you the structure of protonated amino acid.
When the value of pH is higher than isoelectric point.
The presence of hydroxyl ion in the solution is found to be more when the value of pH is more than isoelectric point. This causes excess hydroxyl ions to attract the amine group which results in removal of hydrogen ions. Leaving only negative charge on the carboxylate group, amine has neutral charge on it. In the overall scenario amino acids have charge -1.
Also read :
- NCERT solutions for Class 12 Chemistry Chapter 13 Amines
- NCERT Exemplar Class 12 Chemistry solutions Chapter 13 Amines
- NCERT notes Class 12 Chemistry Chapter 13 Amines
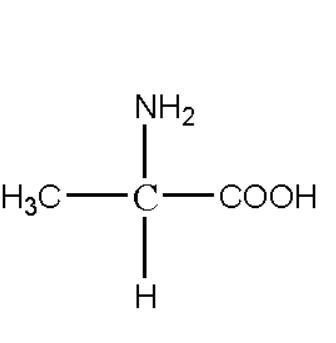
Zwitterion structure of alanine:
The alanine is a form of amino acid which means it contains one carboxylic group and one amino group. While defining the alpha amino acid the alanine is the example of such alpha amino acid group. The alanine is consisting of two carbon atoms where the same carbon atom has both amine and carboxylic group with molecular formulaC3H7NO2. The structure of alanine can be depicted as follows:
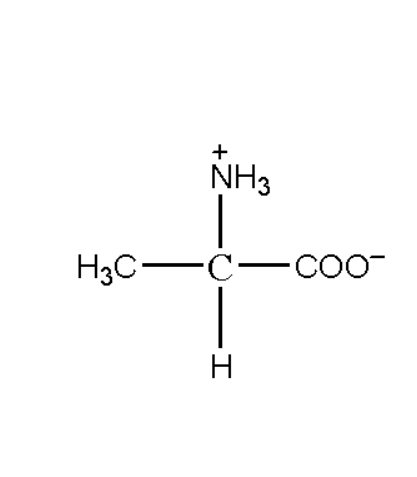
In the above structure we can see that protons from the carboxylic group will shift to the amine group. This happens as the carboxylic acid has a tendency to accept protons and amine to release the proton. The zwitterion is formed can be depicted as below
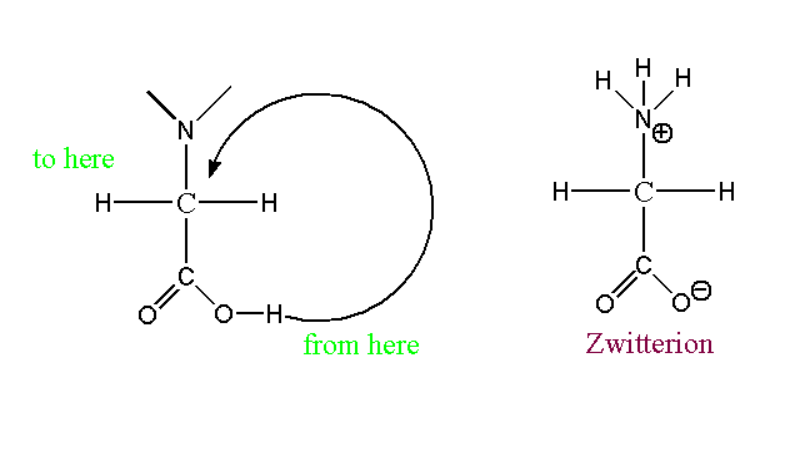
At pH 4 glycine exists as:
The simplest form of amino acid is glycine which can be represented by the symbol G or Gly. It is a form of amino acid which has a hydrogen atom in its side chain. Glycine is the only amino acid that can exist as achiral proteinogenic amino acid and can be fitted in both the environment as hydrophilic and hydrophobic. This happens because of one hydrogen atom.
At pH 4 glycine is found as in acidic form.
Zwitterion structure of glycine:
The zwitterion structure can be represented at this pH range.
When reacts with hydrogen ion such reaction can proceed further:
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes :
Frequently Asked Questions (FAQs)
Isoelectric point pH can be defined as a particular pH range molecule carrying no net charge on it. The net charge is affected by the pH and its surrounding.
Zwitterion is composed of positively and negatively charged electrons and are neutral in nature. By adding the hydroxide ions the pH increases which ultimately exclude the hydrogen ions from the amine group.
The best example of zwitterion is amino acids. Amino acids consist of two functional groups: amine group and carboxyl group.
The isoelectric point definition is given by those points where the pH of anion are equal to pH of cation. At this point amino acids do not migrate, when a field is applied on it.
Dipolar ions are those that have both charges positive and negative and are amphoteric in nature.
Zwitterion can be defined as the molecule which is made up of two functional groups. The opposite charges present on the group will cancel out each other making the compound neutral.
Also Read
02 Jul'25 05:14 PM
02 Jul'25 05:12 PM
02 Jul'25 05:11 PM
02 Jul'25 05:10 PM
02 Jul'25 05:03 PM
02 Jul'25 04:57 PM
02 Jul'25 04:56 PM
02 Jul'25 04:51 PM
