Anilines (C6H5NH2) - Formula, Preparation, Structure, Properties, FAQs
After reading this article, the reader should be able to understand- Aniline formula, Aniline Structure, resonating structure of aniline, density of aniline, melting point of aniline, boiling point of aniline.
Aniline
Aniline is the simplest organic compound that is an aromatic benzene with the chemical formula- C6H5-NH2
Aniline is an aromatic amine in which an amine group (-NH2) is attached to a benzene group. Therefore, The Functional group of Aniline is amino group.
| Aniline boiling point- | 184.1⁰C |
| Aniline density- | 1.02 g/cm3 |
| Aniline Melting point- | -6.3⁰C |
Aniline structural formula is shown below-
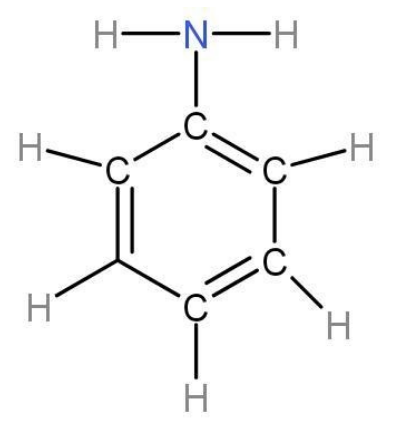
- Structure of aniline- If you take out one hydrogen from ammonia and attach a benzene group with it, it gives us the structure of aniline. Aniline molecular formula is C6H7N and chemical formula is C6H5-NH2
- The molecular weight of aniline is 93 g/mol (12×6 Carbons+7×1Hydrogens+14×1Nitrogen)
- Aniline is an unsaturated compound due to the presence of an unsaturated benzene ring. Unsaturation arises due to bonds other than sigma and Benzene has three pi bonds.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Aniline
- Resonance structure of aniline
- Preparation of aniline-
- Reactions of Aniline-
- Aniline uses-
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Nomenclature of Aniline-
- The General common name for all the aromatic amine is aryl amine.
- IUPAC name for aromatic amine is benzenamine. For ex- Aniline IUPAC name is benzenamine because aniline is the simplest aromatic amine.
- Aniline and benzylamine are two different compounds. Aniline is an aromatic amine however, benzylamine is an aliphatic amine which means amine is not directly attached to an aromatic system in benzylamine.
Physical properties of aromatic amines-
- Aromatic amines are generally toxic.
- Aniline color in pure form is colorless, however on oxidation and addition of impurities they start to impart a certain color.
Isomerism in Aniline-
- Aromatic amine can show position isomerism when another group such as methyl or nitro group are introduced on the benzene ring. The positions of these groups can change in position with respect to amine group.
- Ortho, para and meta are three positions for amine group with respect to methyl. All these three structures will give position isomers of aniline.
Basicity of Aniline-
- Aniline contains one nitrogen which has one lone pair of electrons which determines aniline nature. Nitrogen forms three bonds in total and rest of the two electrons exist as lone pair.
- Lone pair accounts for the basic nature of all nitrogen-containing compounds. However, there can be other factor that can increase or decrease the basicity of a compound.
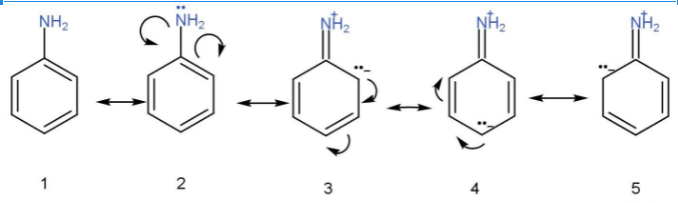
- For ex- ethylamine and aniline are both Nitrogen-containing compounds. However, ethylamine is more basic than aniline. This is because in case of aniline the lone pair is not available for donation as it participates in conjugation. In Structure 3,4, and 5 there is a positive charge on nitrogen atom hence due to resonance aniline becomes less basic due to resonance.
- These electrons interact with the pi system of benzene ring and resonating structures are obtained.
- Aniline resonance is due to the Resonating structure of aniline a
Resonance structure of aniline
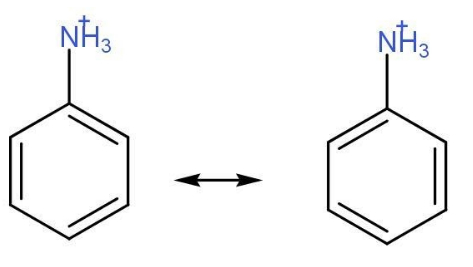
- Anilinium ion- This ion is formed after the Amine group attached to benzene group gets protonated and nitrogen acquires a positive charge. The resonating structures for this ion are only two and we know the more is the number of resonating structures more is the stability of that compound.
Resonating structure of Anilinium ion-
Preparation of aniline-
A variety of substrates of are used to synthesize aniline such as alkyl halides, nitro groups, nitriles, amides, benzamides, and benzoic acid.
- Alkyl halides-
Nucleophilic substitution reaction of ammonia on alkyl halides takes place at 373 K in a sealed tube. An ammonium salt is formed which reacts with a base to form primary amine.
RX+NH3→RNH3+X-
RNH3+X- + NaOH→RNH2+ H2O+NaX
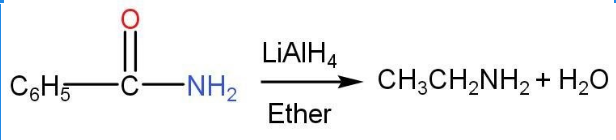
- Amides-
Amides undergo reduction in the presence of lithium aluminium hydride. The number of carbon atoms are same as the original amide.
- Carboxylic acid -
Carboxylic acids are used to synthesize primary amines in the presence of hydrazoic acid.
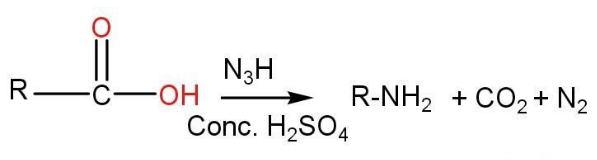
- Nitro compounds-
Nitro compounds get reduced in the presence of acid and active metals such as zinc, iron, and tin.
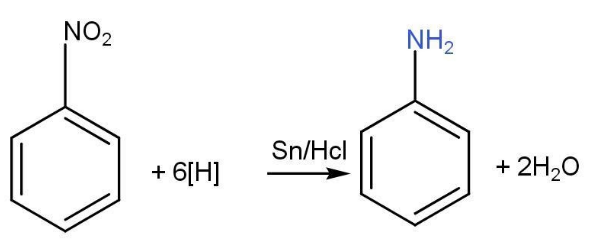
- However, on an industrial scale the best method for the production of aniline is the catalytic hydrogenation of nitrobenzene by hydrogen in the presence of platinum.
- Few other known methods for the synthesis of aniline are by the treatment of chlorobenzene with ammonia at 473 k and a pressure of 60 atm pressure.
Reactions of Aniline-
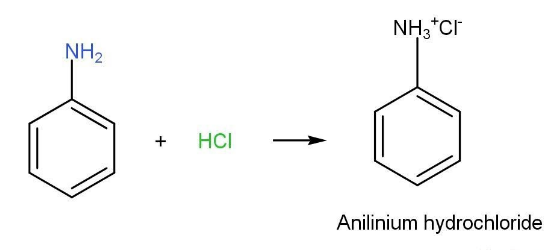
- Reaction with acid-
Due to the presence of lone pair on nitrogen aniline behaves as basic in nature. Anilinium hydrochloride is the product that is formed. If a strong base is added, then aniline can be liberated.
Aniline HCl reaction is shown as following-
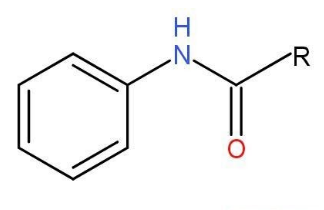
- Acylation- Benzenamine reacts with acetyl chloride in the presence of base such as pyridine to form analide. A hydrogen group of -NH2 is replaced with
–RCO group. The presence of base is necessary for the removal of Cl of acid chloride. Base should be stronger than ammonia to shift the equilibrium to the right side of the reaction.
Analide formula is N- Phenyl ethanamide or acetanilide.
Analide structure is shown below-
Related Topics Link, |
Alkylation of aniline-
It is possible to introduce a methyl group onto the nitrogen atom of -NH2 group by the removal of Hydrogen. An alkyl halide is introduced for the nucleophilic substitution reaction. Here the amine will act as nucleophile and alkyl halide performs the attack.
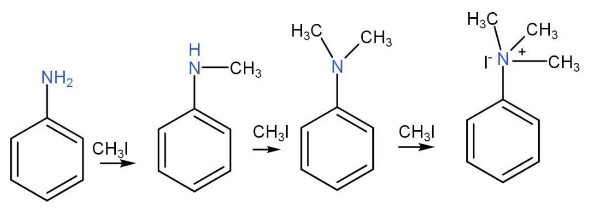
Oxidation of Aniline-
Oxidation of amines depend upon their structure as well as the selected oxidizing agent. Many different types of oxidizing agents are used like Caro’s acid, hydrogen peroxide, potassium permanganate etc.
Eg- Potassium permanganate-
RH2 [o]→ RNHOH [o]→ R-N=O [o]→ R-NO2
Also read :
- NCERT solutions for Class 12 Chemistry Chapter 13 Amines
- NCERT Exemplar Class 12 Chemistry solutions Chapter 13 Amines
- NCERT notes Class 12 Chemistry Chapter 13 Amines
Aniline uses-
Aniline is a crucial raw material for the synthesis of many industrial products such as polymers, pesticides, paracetamol, rubber and dyes.
Aniline polymerization-
Polymer of aniline is a conducting polymer. PANI is the polymer of aniline. All the monomer units of polyaniline are arranged in a head-to-toe manner.
Polyaniline structure is a stable structure due to strong stabilizing agents such very strong acids.
Synthesis-
Aniline polymerization is shown as follows-
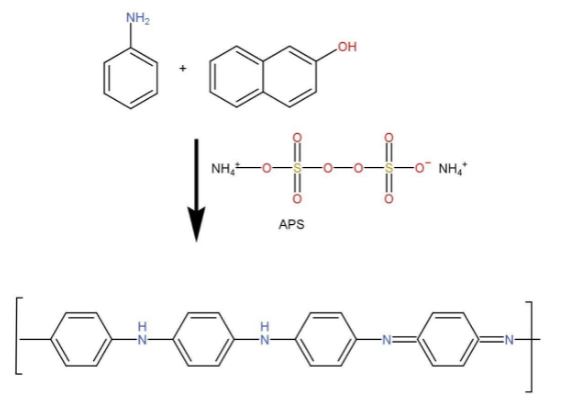
Polyaniline properties help in production of colored electrically conductive products, formation of plastic batteries and photovoltaic cells.
- Aniline oil- is used in formation of dyes and medicines.
Anisole structure
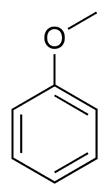
Anisole IUPAC name
Anisole IUPAC name is methoxybenzene.
Pentyne structure
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes :
Frequently Asked Questions (FAQs)
In anthranilic acid, benzene acts as electron withdrawing group that depresses the tendency of weak acidic group (-COOH) to donate its proton to amine -NH2 group. Whereas, in case of aliphatic chains for ex- glycine can exist as zwitter ion.
Aniline point is a measure of miscibility of lubricating oils with aniline when the volumes of aniline is equal to other lubricating oils.
Aniline is an unsaturated compound due to the presence of benzene ring. Benzene has three double bonds and these Double bonds make a compound unsaturated.
Aniline is converted to Diazonium salt. Diazonium salt is further converted to benzonitrile.
Aniline is an aromatic amine with a chemical formula of -C6H5NH2. A benzene group attached to a -NH2 group is called aniline.
-OCH3 group is electron releasing group release the electrons and increases the basic strength of aniline.
Also Read
02 Jul'25 05:14 PM
02 Jul'25 05:12 PM
02 Jul'25 05:11 PM
02 Jul'25 05:10 PM
02 Jul'25 05:03 PM
02 Jul'25 04:57 PM
02 Jul'25 04:56 PM
02 Jul'25 04:51 PM