Schottky Defect - Definition, Examples, Diagram, Formula, Characteristics, FAQs
What is Schottky Defect?
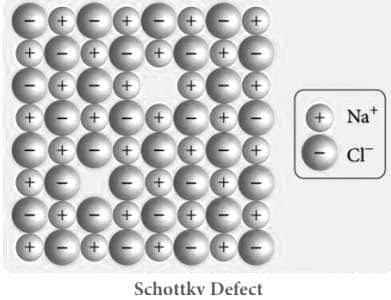
When an equal number of cations and anions are missing from the lattice, a Schottky defect occurs. Lattice structures (also known as crystals) are far from flawless, just like the human body. Our bodies try hard to make things proportionate, but sometimes our right foot is bigger than our left; similarly, crystals try to organise their ions in a tight arrangement, but sometimes an ion slips to another position or just disappears.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Schottky Defect?
- Explain schottky defect with Schottky Defect diagram
- Schottky Defect Characteristics
- Schottky Defects Formula
- Schottky Defect Examples
- Differentiate Between Frenkel and Schottky Defects?
Realistically, it is to be expected that crystals will deviate from their normal sequence (not surprising considering defects occur at temperatures greater than 0 K). There are numerous ways for a crystal to lose its order (and hence develop faults); these defects are classified as Point Defects, Line Defects, and other types of defects.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Explain schottky defect with Schottky Defect diagram
Schottky Defects in Specific Areas
Point defects in lattice structures (or crystals) can be one of two types:
atoms or ions that have left their original location (thus creating vacancies).
Interstitials are formed by atoms or ions slipping into the small gaps between other atoms or ions; as atoms or ions in crystals occupy interstitials, they intrinsically become (make) interstitials.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 1 The Solid State
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 1 The Solid State
Schottky Defect Characteristics
When heat is applied to crystals of ionic substances, Schottky defect forms. Thermal oscillations occur inside the crystal as the temperature rises due to the heat. As a result, gaps appear in the crystal pattern. The availability of ions in chemical compounds causes voids to form.
The "n" ions of X and the "m" ions of Y, for example, will exit the lattice to generate a vacancy in an ionic compound with the formula XnYm. A Schottky cluster is a collection of these job openings.
The following are some properties of the Schottky Defect:
The size of the anion and cation differs by a little amount.
In most cases, two vacancies
Schottky Defects Formula
Heat is used to create Schottky defects in solid crystals. The following formula can be used to compute the faults at a given temperature:
Ns×≈Ne -Hs/2RT
Were,
At temperature T, ns = number of Schottky defects per unit volume (in Kelvins)
Hs is the enthalpy of producing a single flaw.
R is the gas constant.
T stands for absolute temperature (in K)
The following formula can be used to calculate N:
N= (Ionic Crystal Compound Density NA) / (Molar Mass of Ionic Crystal Compound)
Schottky Defect Examples
A Schottky defect is a type of crystal defect that occurs mostly in strongly ionic or highly coordinated substances. The size difference between the anions and the cations in the compound's lattice is quite modest.
Examples of Schottky defect
Sodium chloride (NaCl)
potassium chloride (KCl)
potassium bromide (KBr)
caesium chloride (CsCl) and silver bromide are some examples of salts with Schottky faults (AgBr).
The following formula can be used to calculate N:
N= (Ionic Crystal Compound Density NA) / (Molar Mass of Ionic Crystal Compound)
Related Topics, |
Differentiate Between Frenkel and Schottky Defects?
Despite the fact that both the Schottky and Frenkel defects are point defects that only arise in ionic compounds, there is a significant distinction between them. The following table lists the differences between the Schottky and Frenkel defects.
Schottky Defect
The Schottky defect develops when oppositely charged atoms (cation and anion) leave their lattice locations and produce a pair of Vacancy Defects. A Frenkel Defect occurs when an atom (especially a cation) leaves its initial lattice site and occupies an interstitial position on the same crystal.
Only the minor cation leaves its lattice site in the Frenkel defect, but the anion remains in its lattice site.
Both the anion and the cation leave the solid crystal in the Schottky defect.
The number of atoms in the crystal before and after the Frenkel defect is the same.
Each Schottky defect removes two atoms from the crystal.
The atoms move away from their original lattice location and into an interstitial space.
The atoms leave the crystal permanently.
Because no atom leaves the solid crystal following the Frenkel defect, the density of the solid crystal remains constant.
The Schottky defect reduces the density of the sol by causing vacancy to develop.
Schottky defect in ceramic material is a pair of nearby cation and anion vacancies.
Points to Remember About the Schottky Defect
The Schottky defect is a point defect in which both cation and anion are missing in equal amounts from the lattice site. NaCl, CaCl, and other salts are examples.
Walter H. Schottky, a German physicist, discovered the Schottky Defect, often known as the small shot effect.
When heat is applied to crystals of ionic substances, Schottky defects form.
Only ionic substances have the Schottky and Frenkel defects, which are point defects. When both cation and anion depart their lattice positions and generate a pair of Vacancy Defects, the Schottky defect occurs. When an atom (particularly a cation) departs its initial lattice position and occupies an interstitial space, the Frenkel Defect occurs.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Also Read
02 Jul'25 05:02 PM
02 Jul'25 04:51 PM
02 Jul'25 04:50 PM
02 Jul'25 04:43 PM
02 Jul'25 04:26 PM