Bravais Lattice - Types, Crystal Structures and FAQs
In the year of 1850 scientist name Auguste Bravias discovered about bravais lattice. Bravais lattice generally refers with the different type of arrangement of atoms in a crystal in three dimensional which is generally abbreviated as 3-D geometry. Bravais lattice generally referred to the arrangement of atoms in any crystal in its three dimensional structure and there are 14 known arrangement of bravais lattices are present by now.
Before moving to bravais lattice the first thing we have to know about is unit cell which can be defined as the smallest group of those atoms which are symmetrically aligned and those are repeating repeatedly in an array which further form the entire crystal i.e. in easy manner we can say unit cell is the repeating unit form the whole crystal.
There are a number of methods which easily describe bravias lattice the most common and easiest way to describe what is bravais lattice; Bravias lattice definition can be defined as an arrangement of different points in an array and orientation of these points look exactly same from any point or we can say that lattice points which are of indistinguishable in nature.
Bravais lattice is said to or we can say refer to be one of the 14 different types of unit cells through which a crystal structure is made up of where crystal structure can be defined as any structure which is composed up of atoms or in which atoms are arranged in a definite order while a crystal lattice is made up of points. Bravais lattice is named after the scientist who discovered this, A French scientist named Auguste Bravais.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Bravais Lattice Types
There are basically 14 bravias lattice out of these type of lattice 5 bravais lattice are grouped together in four crystal lattices out of which every crystal lattice is divided into two categories known by the name primitive and centered crystal lattice and these 5 bravais lattice are combined into 4 crystal lattice which can be defined as follows:
1. Monoclinic crystal lattice: In this type of crystal lattice bravais lattice in present in the primitive category and have oblique type structure.
2. Orthorhombic crystal lattice: In this 2 bravais lattice are present in both primitive as well as centered crystal lattice in the form of rectangular and centered rectangular crystal lattice.
3. Tetragonal crystal lattice: In this type of bravais lattice is present in primitive group as square. These can also be present as face centred tetragonal.
4. Hexagonal crystal lattice: In this bravais lattice is present in the form of hexagon in primitive type.
7 lattices are present in three dimensional geometry which can be discussed as follows:
1. Cubic lattice: In this type of crystal lattice the following relationships between unit cells like the letter a, b and c which describe the dimensions of unit cell and letter represented by ![]() denotes the corresponding angle and the relation can be shown as follows:
denotes the corresponding angle and the relation can be shown as follows:
a = b = c and ![]()
In this cubic lattice three possible types of lattices are there which can be known by the name Simple cubic cell or primitive unit cell, Body centered cubic cell and face centered cubic unit cell.
The main cubic lattice example for cubic structure is polonium, for body centered cubic iron is the one and for face centered cubic is copper.
2. Orthorhombic lattice: These are of orthorhombic structure and relation between edge lengths and angles can be derived as:
![]() and
and ![]()
In this type four possible structures can be seen which are named as simple cubic, base centered cubic unit cell, body centered cubic unit cell and face centered cubic unit cell.
The main example of orthorhombic crystal lattice are: For orthorhombic crystal lattice the main example is Rhombic sulfur, base centered orthorhombic structure can be seen in case of magnesium sulfate hexahydrate, potassium nitrate is said to be the example of body-centered orthorhombic while the barium sulfate is the known example for face-centered orthorhombic.
3. Tetragonal systems: In this type of crystal lattice the relation can be discussed as follows:
![]() and
and ![]()
There are only two types of tetragonal unit cell which can be known by the name simple tetragonal cell and body centered tetragonal cells.
Examples of tetragonal bravais lattice are stannic oxide and titanium dioxide for simple tetragonal and body centered tetragonal respectively.
Related Topics, |
4. Monoclinic systems: Bravais lattice which shows monoclinic system can be have the relations of edge length and angles can be shown as follows:
![]() and
and ![]()
Two main possible structures shown by monoclinic structures are primitive and base centered monoclinic unit cells and for these two the main examples are monoclinic sulfur in case of primitive monoclinic system while for base centered monoclinic sodium sulfate decahydrate is the known example.
5. Triclinic system: In this type of system only one bravais lattice is known which is called primitive cell and the relation between edge lengths and the angles of this cell can be shown as follows:
![]() and
and ![]()
Triclinic unit cell are known in the form of potassium dichromate.
6. Rhombohedral system: This type also contain only one type of bravias lattice which can be known by the name rhombohedric primitive unit cell or rhombohedral unit cell in which the relations of edge length and angle can be shown as:
a = b = c and ![]() .
.
The main examples of simple rhombohedric unit cell are calcite and sodium nitrate.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 1 The Solid State
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 1 The Solid State
7. Hexagonal system: This hexagonal system also contain only one type of lattice cell known by the name simple hexagonal cell and the relation of edge lengths and angles can be represented as:
![]() and
and ![]()
The main examples of simple hexagonal unit cells are zinc oxide and beryllium oxide. Other arrangement of this bravias lattices can be shown in four dimensional geometry abbreviated as 4-D geometry. In this 64 types of bravais lattice out of which 23 are known as primitive one and most bravais lattices are of the type i.e. 41 are known by the centred lattice whereas ten bravais lattices are split into enantiomorphic pairs. From all this discussion we can notice that 14 possible bravais lattices are varies from each other with the relationship between edge length and angles as for every arrangement the relation is different. It is also an important thing which suggest that bravias lattice is not exactly equal to crystal lattice in each case. Bravias lattice table can be shown as:
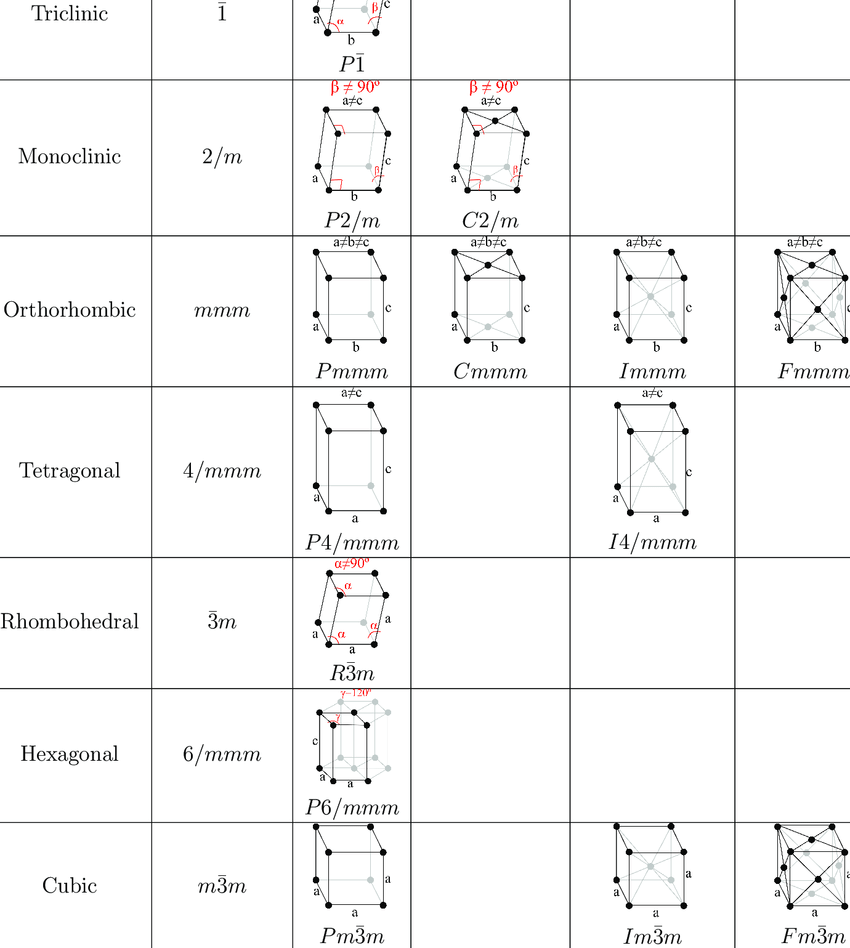
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
There are 14 types of bravias lattice out of which 4 contain more than one crystal lattice which can be named as cubic lattice, orthorhombic lattice, tetragonal systems and monoclinic systems.
In an easy manner we can describe unit cell as the shortest or smallest cell which by repeating itself many times to form a whole crystal lattice.
Bravias lattice was discovered in the year of 1951 by the scientist named Auguste Bravias who was a French scientist and also the name bravias lattice is originated from the name of its discoverer.
The main example of orthorhombic crystal lattice are: For orthorhombic crystal lattice the main example is Rhombic sulfur, base centered orthorhombic structure can be seen in case of magnesium sulfate hexahydrate, potassium nitrate is said to be the example of body-centered orthorhombic while the barium sulfate is the known example for face-centered orthorhombic.
This relation is true for cubic lattice and rhombohedral system.
Also Read
02 Jul'25 05:02 PM
02 Jul'25 04:51 PM
02 Jul'25 04:50 PM
02 Jul'25 04:43 PM
02 Jul'25 04:26 PM

