Newlands Law Octaves - Limitations, Advantages, FAQs
Early attempts at grouping components into groups based on their qualities included Dobereiner's triads and Newland's law of octaves. The broad division of elements into metals and non-metals became ineffective when many new elements were found during the 18th and 19th centuries. Several studies were carried out in order to locate and group elements having comparable qualities. It is indeed worth noting that early techniques of classifying elements, such as Newland's law of octaves and Dobereiner's triads, set the groundwork for the contemporary periodic table's growth.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What are Dobereiner's Triads
- Triad 1
- Triad 4
- Triad 5
- Limitations of Dobereiner's Triad
- Advantages of Dobereiner's Triad
- Limitations of Newlands Law of Octaves
- Advantages of Newlands Law of Octaves
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What are Dobereiner's Triads
Johann Wolfgang Dobereiner, a German scientist, developed Dobereiner triads, which are groups of elements with identical properties. He also noticed that triads (groups of three elements) could be constructed, with all of the components having the same physical and chemical properties.
 Dobereiner - German Chemist
Dobereiner - German Chemist
According to Dobereiner's law of triads. He also proposed that this law be extended to include other quantitative qualities of elements, such as density.
Dobereiner's initial triads, discovered in 1817, consisted of the alkaline earth metals strontium, barium, and calcium. By 1829, three more triads had been recognised, and these triads are listed below:
Triad 1
The alkali metals lithium, sodium, and potassium formed this triad.
TRIAD | ATOMIC MASS |
LITHIUM | 6.94 |
SODIUM | 22.9 |
POTASSIUM | 39.1 |
The arithmetic mean of the masses of potassium and lithium is 23.02, which is nearly equal to atomic mass of sodium.
Read more :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Triad 2
TRIAD | ATOMIC MASS |
CALCIUM | 40.1 |
STRONTIUM | 87.6 |
BARIUM | 137.6 |
The arithmetic mean of the atomic masses of barium and calcium corresponds to 88.7.
Triad 3
TRIAD | ATOMIC MASS |
CHLORINE | 35.4 |
BROMINE | 79.9 |
IODINE | 126.9 |
The average of the atomic masses of chlorine and iodine is 81.1 which is nearly equal to that of bromine.
Related Topics Link, |
Triad 4
The elements sulphur, selenium, and tellurium forms the fourth triad.
TRIAD | ATOMIC MASS |
SULPHUR | 32.1 |
SELENIUM | 78.9 |
TELLURIUM | 127.6 |
The average of the masses of sulphur and tellurium is 79.85 close to that of selenium.
Triad 5
The final of Dobereiner's triads was iron, cobalt, and nickel.
TRIAD | ATOMIC MASS |
IRON | 55.8 |
COBALT | 58.9 |
NICKEL | 58.7 |
The mean of the masses of iron and cobalt correspond to 57.3.
Limitations of Dobereiner's Triad
The following are the major flaws in Dobereiner's approach of classifying elements.
This model was rendered outdated by the discovery of additional elements.
The triads did not fit newly found elements.
Only five Dobereiner's triads were discovered.
Several well-known elements were unable to fit into any of the triads.
Other techniques of classifying elements were created as a result of these flaws.
Advantages of Dobereiner's Triad
The known elements could not be arranged in form of triads. For very low mass or for very high mass elements, law was not holding good. Also it made chemist look at elements in terms of group of elements with similar chemical as well as physical properties.
Newlands Law of Octaves
 John Newlands
John Newlands
John Newlands, a British scientist, attempted the 62 elements known at the time in 1864. He organised them in ascending order based on their atomic weights and discovered that the attributes of every eighth element were the same. Newland's law of octaves was created as a result of this finding.Newland’s law of octaves - When the elements are organised in ascending order of their atomic masses, the law of octaves states that every eighth element has comparable properties.
Related Topics Link, |
Below is an illustration of the elements with comparable qualities according to Newland's law of octaves.
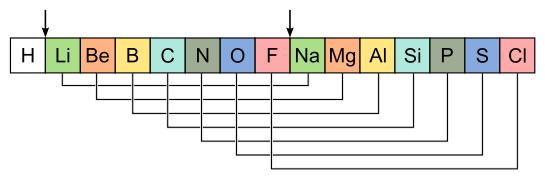
Newlands contrasted the components' closeness to musical octaves, in which every eighth note is equivalent to the first. This was the first time an atomic number was assigned to each element. However, in the scientific world, this approach of classifying elements was received with a lot of opposition.
NCERT Chemistry Notes:
Limitations of Newlands Law of Octaves
The following are the major flaws in Newland's law of octaves.
Newland could only organise elements up to calcium out of the entire 56 known elements.
In Newland's periodic classification, some elements were grouped together. Cobalt and nickel, for example, were placed in the same slot.
Element properties that were dissimilar were grouped together. Halogens, for example, were classified alongside metals like cobalt, nickel, and platinum.
Only up to calcium did Newland's law of octaves hold true. The atomic masses of elements with higher atomic masses could not be accommodated in octaves.
Later discovered elements could not be incorporated into the octave pattern. As a result, this system of classifying elements left no possibility for new elements to be discovered.
Noble gases were not found at the time, hence they were not included in the periodic table.
Advantages of Newlands Law of Octaves
The following are some of the benefits of Newlands' Octaves law:
This law establishes a framework for classifying elements with comparable features into groups.
All the elements were arranged in a tabular format
Newlands law of octave was the first to classify based on the atomic weight, linking the properties of the elements to their atomic masses.
For the lighter elements, this system performed much better. Lithium, sodium, and potassium, for example, were put together.
Also check-
Frequently Asked Questions (FAQs)
Calcium, strontium and barium is an example for Dobereiner's triad.
Newlands law of octaves was proposed by John Alexander Newlands
Calcium (Ca), strontium (Sr), and barium (Ba), all of which have atomic masses of 40, 88, and 137, respectively, constitute a triad because their chemical properties are similar, and strontium's atomic mass is roughly equal to the average of calcium and barium's atomic masses.
It enables scientists to predict the properties of elements and their compounds based on their positions in the periodic table. It becomes easier to study, comprehend, compare, and contrast the relative properties of elements and their compounds from various groups.
The Law of Octaves only applied up to calcium, because after then, no eighth element has qualities that are identical to the first.
Also Read
02 Jul'25 06:01 PM
02 Jul'25 05:59 PM
02 Jul'25 05:59 PM
02 Jul'25 05:58 PM
02 Jul'25 05:58 PM
02 Jul'25 05:58 PM
02 Jul'25 05:53 PM
02 Jul'25 05:35 PM
02 Jul'25 04:44 PM

