Methanol - Properties, Structure, Occurrence, Uses with FAQs
Methanol is generally said to be an alcohol where alcohol group is represented by the formula OH and ends with the subscript ol. Therefore, it is said to be an alcohol, methanol ended with the word ol.
Methanol chemical formula is CH3OH. Where CH3 the methyl group linked with the hydroxyl group. Methanol is not said to be a hydrocarbon as hydrocarbons only contain hydrogen and carbon atoms while methanol contains oxygen atom also or we can say that in methanol hydroxyl group is chemically bonded with the carbon atom.
- Physical properties of methanol:
- Nature of methanol
- Uses of methanol
Methanol is also known as wood alcohol or methyl alcohol. Methanol consists of a distinctive odour and somewhat milder and sweeter as compared to ethanol. Methanol is generally colorless in nature and of volatile nature. It is a flammable, light and poisonous liquid. Methanol is said to be of toxic nature when it is consumed by humans then it can cause blindness too. The main use of methanol is in the manufacturing of acetic acid and formaldehyde.
Methanol was first isolated in the year of 1661 by the scientist Robert Boyle. Methanol was produced through the distillation of boxwood also known by the name buxus. This is the old method of producing methanol while nowadays methanol is prepared by the direct combination reaction in which carbon monoxide gas combines with hydrogen gas and this reaction further proceeds in the presence of a catalyst. Methanol is a very common solvent used in laboratories. It is also used in the manufacturing of ethanol and acts as a denaturant additive in that process.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Physical properties of methanol:
Methanol is generally present in liquid form which is colorless and volatile in nature. Methanol formula is CH3OH. Molecular mass of CH3OH or methanol molecular weight is given by 32.04 g/mol. Density is 792 kg/m3 and methanol boiling point and melting points can be considered as 64.7ºC and, -97.6ºC, respectively.
Structure
Methanol is also known by the name methyl alcohol so methyl alcohol structure or we can say methanol structure or methanol structural formula is shown as follows:

The three-dimensional form of methyl alcohol can also be seen which is as follows:
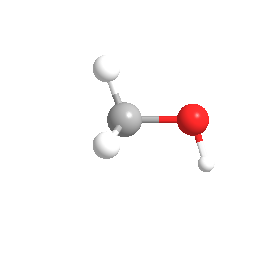
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11 Alcohols, Phenols and Ethers
- NCERT notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
Nature of methanol
Methanol is of basic as well as of acidic nature where basic are those substances which are capable of accepting the protons while acids are those which are capable of donating the protons. Here methanol both proton donors and proton acceptors are present where proton donors are acidic according to Bronsted-Lowry concept and proton acceptors are said to be base. In usual methanol is said to be proton donor as it readily donates O-H proton to some heavy bases like sodium hydride etc.
Occurrence
Methanol in small amounts is present in every normal and healthy human being. Study shows that by inhaling the oxygen a normal person can also inhale 4.5 ppm of methanol along with the oxygen. Mean value of endogenous methanol found in humans is 0.45 g/d which may be metabolized from pectin which is found in fruits. The most in taking fruit apple contains 1.4 g of methanol per kg.
It is also found in some regions of space in the year of 2006, astronomers by using merlin array of radio telescope at Jodrell Bank Observatory found a large cloud of methanol in space while in year 2016 astronomers also found methanol in a planet forming disc around the star named TW Hydrae by using radio telescope named ALMA.
Manufacturing of methanol
Methanol can be manufactured by treating carbon monoxide with hydrogen in the presence of catalyst which further produces methanol and the main catalyst used during this process are mixture of copper and zinc oxide and reaction can be shown as follows:
![]()
In the manufacture of synthesis gas from methane, three moles of hydrogen are produced for every mole of carbon monoxide, whereas the synthesis utilizes only two moles of hydrogen gas for every mole of carbon monoxide. Carbon dioxide injection into the methanol synthesis reactor, where it, too, reacts to generate methanol according to the equation:
![]()
It can also be produced by the biosynthesis process. Methane can be converted into methanol by catalytic conversion which is further affected by enzymes which include methane monooxygenases. This type of enzymes are known as mixed function oxygenases where oxygenation is done with the combination which produces water and NAD+ where NAD is known as Nicotinamide adenine dinucleotide. Reaction of this can be shown as:
![]()
Related Topics, |
Uses of methanol
There are many applications of methanol in industry as well as in daily life, these uses can be explained as follows:
1. Methanol is used in the formation of polymers when they are converted into formaldehyde.
2. One of the important uses of methanol is to produce hydrocarbons.
3. Methanol can also be used as a precursor for methyl ethers, methylamines and methyl halides.
4. Methanol is also useful in the automobile industry; it is used as a fuel in internal combustion engines.
5. This is used as one of the excellent energy carriers.
6. Methanol can also be used in wastewater plants.
7. Methanol is used as fuels in boating stoves and camping.
8. Methanol is also used as an anti-freezing reagent.
9. Another important use of methanol is these are used in the synthesis of a number of chemicals.
10. Pure form of methanol is used in the manufacturing of perfumes, resins and other pharmaceuticals due to its odour.
Other than these uses it is also used as an ingredient in many consumer products like glass cleansers and as a thinner in paints. Methanol is also used for drinking purposes. There are also many harmless methanol-containing products available in the market which can be consumed by humans. Methanol is also present in fruit juices and in those spirits which are distilled naturally.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Chemical name of CH3OH is methyl alcohol as it contains an alcoholic group which is represented by OH.
Methanol is a highly dangerous and combustible substance. Direct intake of more than 10mL can result in lifelong blindness, poisoning of the central nervous system, coma, and death. Inhaling methanol vapours has similar dangers.
Methanol is flammable in nature and present in the form of liquid which is colorless in nature and this contains odor which is somewhat similar to ethanol but not as strong so it will be used for making perfumes.
Methanol is said to be of basic as well as of acidic nature where basic are those substances which are capable of accepting of the protons while acids are those which are capable of donating the protons. Here methanol both proton donors and proton acceptors are present where proton donors are acidic according to Bronsted-Lowry concept and proton acceptors are said to be base. In usual methanol is said to be proton donor as it readily donates O-H proton to some heavy bases like sodium hydride etc.
Also Read
02 Jul'25 05:25 PM
02 Jul'25 05:24 PM
02 Jul'25 04:58 PM
02 Jul'25 04:55 PM
02 Jul'25 04:54 PM
02 Jul'25 04:54 PM
02 Jul'25 04:54 PM

