Classification of Colloids - Types of Colloids, Definition, FAQs
Have you ever wondered how substances like milk, ink, or even fog form? Looking at them, it doesn't seem like they are a mixture of two substances - but they actually are. These are examples of colloids, where particles are dispersed within another substance but are not large enough to settle out or be filtered. Colloids are a type of mixture in which one substance (called the dispersed phase) is finely distributed throughout another substance (called the dispersion medium). Colloidal particles are intermediate in size—larger than molecules in a solution but smaller than particles in a suspension.
- What Is a Colloid?
- Dispersion Medium And Dispersed Phase
- Classification Of Colloids
- Types Of Colloids
- Multimolecular Colloids
- Macromolecular Colloids
- Associated Colloids
- Lyophilic Colloids
- Lyophobic Colloids
- Some Solved Examples
In this article, we will discuss the classification of colloids, the dispersion phase, and the dispersion medium, types of colloids, and some solved examples related to them. To know more about colloids, scroll down.
What Is a Colloid?
A colloid is a heterogeneous mixture in which minute particles of one component are scattered in a dispersion medium of another substance.
The tiny particles in this combination range in size from 1 to 1000 nanometres in diameter, but they remain suspended and do not settle to the bottom. They can be seen with an optical or electron microscope (smaller particles).
A dispersed phase and a dispersion medium make up colloids. They're divided into groups based on the features of the dispersed phase and the medium. Let us discover more about them right now.
The following is the IUPAC definition of colloid:
“A colloidal state is a condition of dispersion in which molecules or polymeric particles with at least one dimension between 1 nanometre and 1 micrometre are distributed in a medium.”
Dispersion Medium And Dispersed Phase
Definition of colloidal dispersion
A colloid is a mixture in which a fine-particle-containing substance (dispersed phase) is combined with another component (dispersion medium). The colloids' particles range in size from 1 to 1000 nm in diameter. Because the particles of the solution do not mix or settle down, the solution is called a colloidal dispersion. In the solution, they are disseminated.
Definition of dispersed phase
The dispersed phase refers to the compounds that are distributed in the solution, whereas the dispersion medium refers to the solution in which they are dispersed.
Depending on the state of dispersion and the medium of dispersion
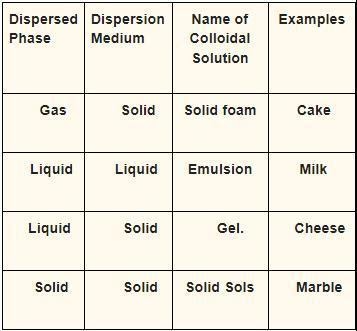
The following classification of colloid systems can be used depending on the state of dispersed particles and the dispersion medium.
- When the Dispersion Medium Is Liquid Foams – When the dispersion medium is a liquid foam. Whipped cream, shaving cream, and other similar products are examples.
- When the dispersed phase is liquid, it is called an emulsion. Milk, mayonnaise, and other foods are examples.
- When the scattered phase is solid, it is called sol. Blood, pigmented ink, and other materials are examples.
- When there is a gaseous dispersion medium
- When the dispersed phase is liquid, it is referred to as a liquid aerosol. Fog, mist, hair sprays, and other similar products are examples.
- When the dispersed phase is solid, it is referred to as a solid aerosol. Smoke, ice clouds, and more examples come to mind
Colloids are classified
When the dispersed medium is a gas, it is referred to as solid foam. Styrofoam, pumice, and other materials are examples.
When the distributed media is liquid, it is called a gel. Agar, gelatin, and other similar substances are examples.
When the scattered medium is solid, it is called solid sol. Cranberry glass is one example.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 5 Surface Chemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 5 Surface Chemistry
- NCERT notes Class 12 Chemistry Chapter 5 Surface Chemistry
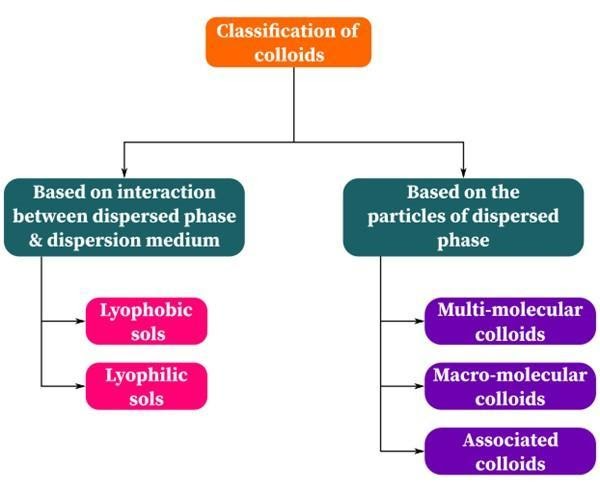
Classification Of Colloids
Colloids are classified based on the nature of the interaction between the dispersed phase and the dispersion medium.
Colloids that are hydrophilic: These are colloids that like to be in the water. The water attracts the colloidal particles. Reversible sols are another name for them. Agar, gelatin, pectin, and other similar substances are examples.
Hydrophobic colloids are the polar opposites of hydrophilic colloids. The water repels the colloid particles. Irreversible sols are another name for them. Gold sols, clay particles, and other materials are examples.
Based on the Dispersed Phase Particle Type
Colloidal solutions can be categorised into three categories based on how different chemicals creating colloidal solutions acquire particle sizes in this range.
Types Of Colloids
The classification of colloids is done based on the types of colloids. The following are the categories:
- Multimolecular colloids
- Macromolecular colloids
- Associated colloids
Multimolecular Colloids
A solution created by the aggregation of a high number of atoms or tiny molecules (with diameters less than 1 nm) in a dispersed medium. Van der Waal forces hold the dispersed particles together.
Example:
Sulphur sol, and gold sol.
|
Related Topics Link, |
Macromolecular Colloids
Molecules with extremely high molecular weights combine to form macromolecules, which are exceedingly large molecules. The resulting colloidal solutions are known as macromolecular colloids when such compounds are dispersed in a suitable dispersion medium. As a result, macromolecular colloids have a large molecular mass. Lyophilic colloids are often macromolecular in nature.
Natural macromolecules, including starch, proteins, gelatin, cellulose, nucleic acids, and others, as well as synthetic polymers like polyethylene, polypropylene, and synthetic rubber, create macromolecular colloids when dispersed in suitable solvents.
Associated Colloids
When present in low quantities, some compounds behave as strong electrolytes, yet when present in high concentrations, they behave as colloidal sols. Particles agglomerate and demonstrate colloidal behaviour at greater concentrations. Micelles are microscopic clumps of collected particles. Associated colloids are another name for them. Micelles are formed above a certain temperature, known as the Kraft temperature (Tk), as well as a certain concentration, known as the critical micelle concentration. By diluting the colloids, they can be converted. Soaps and synthetic detergents are a couple of examples of related colloids.
Classification of colloids based on the interaction medium
Colloids can also be classed according on how the dispersed phase interacts with the medium:
Hydrophilic colloids: Colloids that love or are attracted to water are known as hydrophilic. Reversible sols are another name for them.
Agar, gelatin, and pectin are some examples.
Hydrophobic Colloid: These are the polar opposite of hydrophobic and repel water. Irreversible sols are another name for these.
Gold sols with clay particles, for example.
Colloids are characterised as lyophilic or lyophobic depending on the nature of the interaction between the dispersion medium and the dispersed phase.
Lyophilic Colloids
A lyophilic colloid is one in which the dispersed phase has an affinity for the dispersion medium. The words lyo and philic, respectively, signify "liquid" and "loving." Even if the dispersed phase and the dispersion medium are separated, they can easily be reconstituted by simply mixing them together. Furthermore, because of their sturdy nature, they are difficult to coagulate. Intrinsic colloids are another name for them. Starch, rubber, protein, and other materials are examples.
Lyophobic Colloids
A lyophobic colloid is one in which the dispersed phase has little or no affinity for the dispersion medium. The words lyo and phobic, respectively, denote "liquid" and "fear." As a result, they despise liquids. Because the dispersed phase does not readily form a colloid with the dispersion medium, they are difficult to manufacture and necessitate the employment of special techniques. They are brittle and require stabilising substances to stay alive. Extrinsic colloids are another name for them. Sols of metals such as silver and gold, as well as sols of metallic hydroxides, are examples.
Some Solved Examples
Question.1 Which of the following is an example of a colloidal solution?
a) Salt solution
b) Air
c) Milk
d) Sand in water
Solution:
Milk is a colloidal solution where fat droplets are dispersed in water. The particles in milk are of colloidal size and do not settle out easily, making it a true colloid. Salt solution is a true solution, air is a mixture of gases (not colloidal), and sand in water is a suspension.
Hence, the correct answer is option (c)
Question.2 Which of the following is true for colloidal particles?
a) They are smaller than molecules.
b) They settle under the influence of gravity.
c) They are visible to the naked eye.
d) They cannot be separated by filtration.
Solution:
Colloidal particles are larger than molecules but smaller than particles in a suspension. They do not settle under gravity and cannot be separated by simple filtration, but they can be separated by methods like ultrafiltration.
Hence, the correct answer is option (d)
Question.3 What is the main characteristic that distinguishes a colloid from a solution?
a) Colloids are heterogeneous, while solutions are homogeneous.
b) Colloids have larger particles than solutions.
c) Colloids scatter light (Tyndall effect), while solutions do not.
d) All of the above
Solution:
Colloids are heterogeneous mixtures, while solutions are homogeneous. Colloids have larger particles that are small enough to remain suspended but large enough to scatter light, which is known as the Tyndall effect. Solutions have particles that are too small to scatter light effectively.
Hence, the correct answer is option (d)
Practice More Questions With The Link Given Below
Also read -
Frequently Asked Questions (FAQs)
1. Sols (Solid in liquid):
- Paint: Solid pigment particles dispersed in a liquid.
- Ink: Pigment or dye particles dispersed in water or other solvents.
- Blood: Red blood cells (solid) dispersed in plasma (liquid).
2.Gels (Liquid in solid):
- Gelatin: A liquid (water) dispersed in a solid matrix (gelatin).
- Agar-agar: A gel used in laboratories, made by dispersing water in a solid.
- Jelly: A colloidal gel with liquid dispersed in the solid matrix.
3. Emulsions (Liquid in liquid):
- Milk: Fat droplets dispersed in water.
- Mayonnaise: Oil droplets dispersed in water with the help of an emulsifier.
- Butter: Water droplets dispersed in fat.
4. Aerosols (Solid or liquid in gas):
- Fog: Water droplets dispersed in the air.
- Smoke: Solid particles dispersed in the air.
- Hairspray: Liquid droplets dispersed in air.
5. Foams (Gas in liquid or solid):
- Whipped cream: Air dispersed in cream (liquid foam).
- Soap foam: Air bubbles dispersed in a liquid.
- Foam rubber: Air bubbles dispersed in a solid matrix.
6. Solid Foams (Gas in solid):
- Pumice stone: Air pockets trapped within the solid matrix of volcanic rock.
- Aerogels: Solid materials with gas dispersed within them.
Multimolecular colloid particles are small collections of atoms and molecules with a dimension of less than one nanometre. They also have weak van der Waals interactions between particles, as opposed to macromolecular colloids, which have a significant molecular mass. Between macromolecular particles, they have strong chemical connections. Associated colloids differ from multimolecular and macromolecular colloids in that they form aggregated particles at high concentrations, giving them colloidal qualities. Because of their high molecular mass, they behave like macros.
These are the differences between multimolecular and macromolecular colloids.
Associated colloids are micro heterogeneous colloids in which a substance dissolved in the dispersion medium forms the micelles. They operate like a conventional strong electrolyte at low concentrations, but at greater concentrations, they exhibit colloidal features due to the production of aggregated particles.
With related colloids, two terms are used:
Specific Concentration: Micelles can also develop above a certain concentration, known as the critical micelle concentration.
A solution created by the aggregation of a high number of atoms or tiny molecules (with diameters less than 1 nm) in a dispersed medium. Van der Waal forces hold the dispersed particles together.
Multimolecular Example:
Sulphur sol, and gold sol.
A colloidal dispersion is made up of particles that are scattered in a continuous phase of solid, liquid, or gas (solid, liquid or gas). Colloidal particles are defined as particles having at least one dimension ranging from 1nm to 1m. Solid-liquid (suspensions), liquid-liquid (emulsions), gas-liquid (foams), and solid-gas (aerosols) dispersions are the most prevalent colloidal dispersions.
Because colloidal dispersion seeks to reduce surface energy, it is intrinsically thermodynamically unstable. As a result, a colloidal system's stability is inextricably related to a concept of time, which is defined by the process, usage, and/or application involved.
The stability of a colloid refers to its ability to resist aggregation and separation of its phases. Stable colloids maintain their dispersed state over time due to factors like electrical charges on particles, protective coatings, or the nature of the particle-medium interaction.
The Tyndall effect is the scattering of light by colloidal particles. When a beam of light passes through a colloid, the particles scatter the light, making the beam visible. This effect helps distinguish colloids from solutions, which don't scatter light.
A gel is a colloid where a liquid is dispersed in a solid. Gels form when the dispersed particles in a sol link together to create a semi-rigid structure that traps the liquid. Examples include gelatin desserts and silica gel.
Lyophilic colloids have an affinity for the dispersion medium and are more stable. They form spontaneously and are reversible (e.g., protein solutions). Lyophobic colloids have no affinity for the medium, are less stable, require energy to form, and are often irreversible (e.g., gold sol in water).
Foams are colloids where gas bubbles are dispersed in either a liquid or a solid. They're unique because the dispersed phase (gas) is less dense than the continuous phase. Examples include whipped cream (gas in liquid) and styrofoam (gas in solid).
A colloidal system has two phases: the dispersed phase (the particles) and the dispersion medium (the substance in which particles are dispersed). For example, in milk, fat globules (dispersed phase) are suspended in water (dispersion medium).
Brownian motion is the random movement of colloidal particles due to collisions with molecules of the dispersion medium. This constant motion helps keep colloidal particles suspended and contributes to the stability of the colloid by preventing settling.
Particle size in colloids (typically 1-1000 nm) affects properties like stability, appearance, and behavior. Smaller particles generally create more stable colloids with higher surface area-to-volume ratios, influencing reactivity and optical properties like transparency or opacity.
Electrostatic forces contribute to colloidal stability through the electrical double layer around particles. Many colloidal particles carry a surface charge, attracting oppositely charged ions from the medium. This creates a repulsive force between particles, preventing aggregation and maintaining stability.
Adding an electrolyte to a colloidal system can neutralize the charges on colloidal particles, reducing the repulsive forces between them. This can lead to coagulation or flocculation, where particles aggregate and may eventually settle out of the dispersion medium.
Colloids are classified into eight types based on the physical states (solid, liquid, or gas) of the dispersed phase and dispersion medium. Examples include sol (solid in liquid), emulsion (liquid in liquid), and aerosol (liquid or solid in gas).
A sol is a type of colloid where solid particles are dispersed in a liquid medium. Examples include mud (soil particles in water) and some paints (pigment particles in a liquid base).
An emulsion is a colloid where both the dispersed phase and dispersion medium are liquids that don't mix. In contrast, a sol has solid particles dispersed in a liquid. Milk is an emulsion (fat in water), while ink is often a sol (pigment particles in liquid).
In oil-in-water emulsions, oil droplets are dispersed in water (e.g., milk). In water-in-oil emulsions, water droplets are dispersed in oil (e.g., butter). The continuous phase determines the type: water for oil-in-water, oil for water-in-oil.
Aerosols are colloids where either liquid droplets or solid particles are dispersed in a gas, usually air. They can form naturally (e.g., fog, clouds) or artificially (e.g., spray paint, insecticide sprays). Aerosols can be created by dispersing liquids or solids into a gas.
A colloid is a heterogeneous mixture where tiny particles of one substance are dispersed throughout another substance. The particles are larger than molecules but small enough to remain suspended without settling. Examples include milk, fog, and blood.
Colloids are intermediate between solutions and suspensions. In solutions, particles are molecular-sized and fully dissolved. In suspensions, particles are large and settle over time. Colloid particles are small enough to remain suspended but large enough to scatter light, unlike solutions.
The electric double layer is a structure that forms around a charged colloidal particle in a liquid medium. It consists of a fixed layer of oppositely charged ions tightly bound to the particle surface and a diffuse layer of more loosely associated ions. This layer plays a crucial role in colloidal stability by creating repulsive forces between particles.
The DLVO theory (Derjaguin, Landau, Verwey, and Overbeek) explains colloidal stability by considering the balance between attractive van der Waals forces and repulsive electrostatic forces between particles. It helps predict whether particles will aggregate or remain dispersed based on these competing interactions.
Zeta potential is the electrical potential difference between the bulk of the liquid and the stationary layer of fluid attached to the dispersed particle. A high absolute value of zeta potential (positive or negative) indicates greater electrostatic repulsion between particles, leading to increased colloidal stability.
Coagulation is the process where colloidal particles come together to form larger aggregates, often leading to the separation of the colloid into distinct phases. It can be induced by adding electrolytes, changing pH, or applying heat, and is often used in water treatment to remove impurities.
Protective colloids are substances added to a colloidal system to increase its stability. They work by adsorbing onto the surface of colloidal particles, forming a protective layer that prevents them from coming close enough to aggregate. Examples include gelatin in ice cream and casein in milk.
Physical adsorption (physisorption) involves weak van der Waals forces between the adsorbate and the surface, while chemical adsorption (chemisorption) involves the formation of chemical bonds. Physisorption is reversible and doesn't significantly alter the adsorbate, while chemisorption is often irreversible and can change the adsorbate's chemical nature.
Dialysis is a process used to separate colloidal particles from smaller molecules or ions in a mixture. It involves using a semi-permeable membrane that allows smaller molecules to pass through while retaining larger colloidal particles. This technique is used to purify colloids or remove unwanted electrolytes.
Peptization is the process of converting a precipitate into a colloidal sol by adding an appropriate electrolyte or peptizing agent. This agent helps to break down larger aggregates into smaller colloidal particles, often by charging the particles and creating electrostatic repulsion. It's essentially the reverse of coagulation.
The cloud point is the temperature at which a non-ionic surfactant solution becomes cloudy due to the separation of the surfactant from the solvent. At this point, the solution's properties change dramatically. The cloud point is important in applications where temperature stability is crucial, such as in detergents or industrial processes.
Coacervation is a phenomenon where a homogeneous colloidal solution separates into two liquid phases: a dense, colloid-rich phase (coacervate) and a dilute colloid-poor phase. This process is important in various fields, including microencapsulation technology and is believed to play a role in the origin of life theories.
While both processes involve particle aggregation, flocculation typically refers to the reversible aggregation of particles into loose, fluffy masses (flocs) that can often be redispersed. Coagulation generally involves the irreversible fusion of particles into larger, more compact aggregates. Flocculation is often a precursor to coagulation in water treatment processes.
The depletion force is an attractive force between larger colloidal particles in a mixture containing smaller particles or polymers. It arises from the exclusion of smaller particles from the space between larger ones, creating an osmotic pressure difference. This force can lead to aggregation of larger particles, affecting the stability and phase behavior of the colloidal system.
Steric stabilization is a method of preventing colloidal particles from aggregating by attaching large molecules (often polymers) to their surfaces. These attached molecules create a physical barrier that prevents particles from getting close enough for attractive forces to cause aggregation. This method is particularly useful in non-aqueous systems where electrostatic stabilization is less effective.
Pickering emulsions are stabilized by solid particles rather than traditional surfactants. These particles adsorb at the interface between the two immiscible liquids, forming a physical barrier that prevents droplet coalescence. Pickering emulsions can be more stable than conventional emulsions and find applications in food, cosmetics, and pharmaceuticals.
The DLVO theory, named after Derjaguin, Landau, Verwey, and Overbeek, is fundamental in explaining colloidal stability. It considers the balance between attractive van der Waals forces and repulsive electrostatic forces between particles. The theory helps predict whether particles will aggregate or remain dispersed, guiding the design and control of colloidal systems in various applications.
Emulsification is the process of forming an emulsion by dispersing one liquid in another immiscible liquid. It's achieved by applying mechanical energy (e.g., shaking, blending) and often using emulsifiers, which are substances that stabilize the emulsion by reducing surface tension between the two liquids.
Surfactants stabilize emulsions by reducing the surface tension between the two immiscible liquids. They have a hydrophilic (water-loving) head and a hydrophobic (water-fearing) tail, allowing them to form a layer around droplets of the dispersed phase, preventing coalescence and maintaining the emulsion's stability.
Associative colloids, also known as association colloids or micelles, form when surfactant molecules aggregate in solution above a certain concentration (critical micelle concentration). Unlike other colloids, these are reversible and can form or break apart depending on conditions like concentration and temperature.
Temperature can significantly impact colloidal systems. Higher temperatures generally increase Brownian motion, potentially enhancing stability. However, excessive heat can also disrupt the balance of forces maintaining the colloid, leading to coagulation or phase separation in some cases. Temperature changes can also affect the solubility of components and the critical micelle concentration in associative colloids.
Colloidal gold refers to a sol where gold nanoparticles are dispersed in water. It's typically prepared by reducing a solution of gold chloride with a reducing agent like sodium citrate. The resulting gold nanoparticles are stabilized by the citrate ions, creating a stable red or purple colloidal solution widely used in research and medical applications.
Hydrophilic colloids have an affinity for water and tend to be more stable in aqueous environments. They often form spontaneously and can be easily redispersed if dried. Hydrophobic colloids, on the other hand, repel water and are less stable in aqueous media. They typically require more energy to form and maintain, and may not easily redisperse once separated.
The critical micelle concentration (CMC) is the concentration of surfactants above which they spontaneously form micelles (associative colloids) in solution. Below the CMC, surfactants exist as individual molecules. Above the CMC, any additional surfactant forms micelles rather than increasing the concentration of free molecules, significantly changing the solution's properties.
Multimolecular colloids are formed by the aggregation of many small molecules into larger colloidal particles (e.g., gold sol). Macromolecular colloids consist of large molecules that are themselves of colloidal size (e.g., protein solutions). The key difference is in the nature of the dispersed phase: aggregates vs. single large molecules.
Salting out is the process of reducing the solubility of a substance in a solution by adding an electrolyte. In colloidal systems, high concentrations of salt can neutralize the charges on colloidal particles, reducing their stability and causing them to aggregate and precipitate out of solution. This effect is used in protein purification and other industrial processes.
Amphiphilic molecules, having both hydrophilic and hydrophobic parts, play a crucial role in forming and stabilizing certain colloids, especially emulsions and foams. They can orient themselves at interfaces, reducing surface tension and preventing coalescence of dispersed particles or droplets. This property makes them effective emulsifiers and foaming agents.
Ostwald ripening is a phenomenon where smaller particles in a colloid dissolve and redeposit onto larger particles over time. This process is driven by the system's tendency to minimize surface energy. It can lead to the growth of larger particles at the expense of smaller ones, potentially destabilizing the colloidal system.
The Hofmeister series ranks ions based on their ability to salt out or salt in proteins and affects colloidal stability. Some ions (e.g., sulfate) tend to increase surface tension and promote protein precipitation (salting out), while others (e.g., iodide) tend to decrease surface tension and increase protein solubility (salting in). This series helps predict ion effects on colloidal stability in various systems.
Creaming is a form of emulsion instability where dispersed droplets rise to the top of the continuous phase due to density differences. It's often seen in oil-in-water emulsions where oil droplets are less dense than water. While not a complete breakdown of the emulsion, creaming is a step towards phase separation and indicates reduced stability.
Also Read
07 Aug'25 01:49 PM
04 Aug'25 10:45 AM
02 Jul'25 06:34 PM
02 Jul'25 06:34 PM
02 Jul'25 06:05 PM
02 Jul'25 06:05 PM
02 Jul'25 06:05 PM
02 Jul'25 06:04 PM
02 Jul'25 06:04 PM
02 Jul'25 05:20 PM