Acetylsalicylic Acid - Preparation, Structure, Properties & Uses, FAQs
Acetylsalicylic acid is a small fragrant acid with its chemical name monohydroxy benzoic acid. It is naturally lipophilic and was first taken from the bark of the Willow Tree. It derives its common name from various related sources with the same name, e.g. It is available as a product of salicin (β-glycoside alcohol found in plants) and is an active metabolite produced from acetylsalicylic acid (aspirin). Naturally, they form like clear and colourless crystals of organic acid. The salt content and ester of this substance are also widely used in living chemicals and are known as salicylates.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Preparation of Aspirin or Acetylsalicylic acid
- Physical Properties of Acetylsalicylic acid
- Chemical Properties of Acetylsalicylic acid (Acetylsalicylic acid Reaction) / What is aspirin?
- Methods of Preparation Acetylsalicylic acid
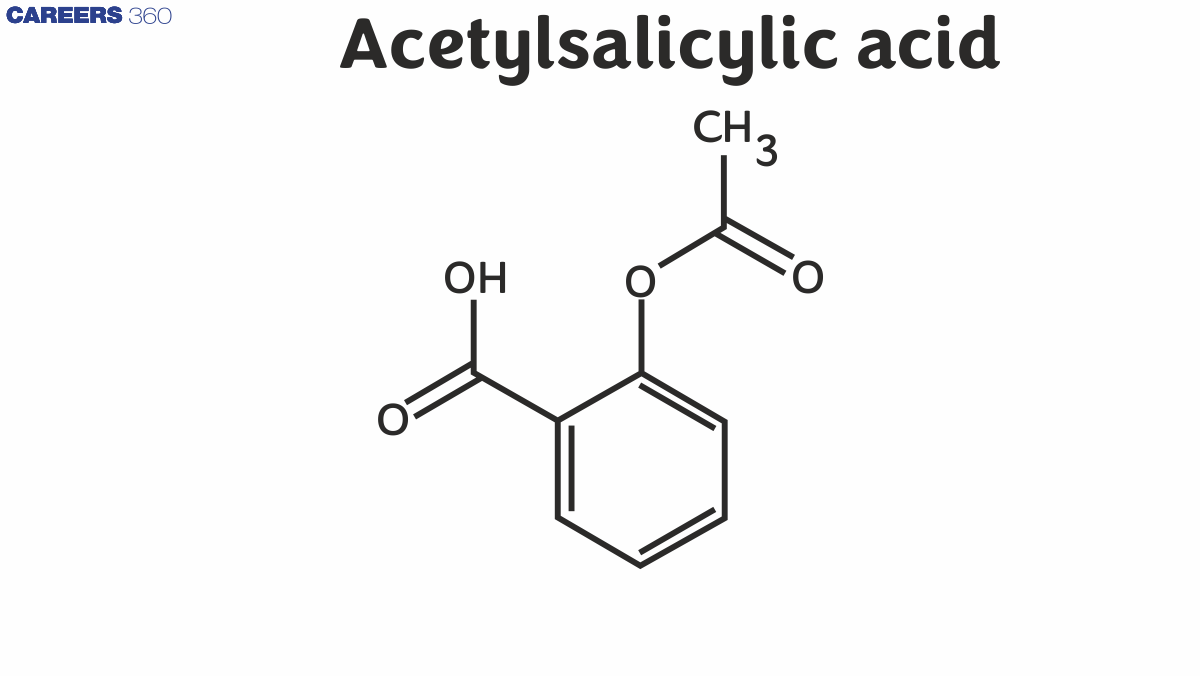
Preparation of Aspirin or Acetylsalicylic acid
IUPAC name of aspirin C6H4 (OH) COOH, which can also be written as C7H6O3 in a concise manner. Its IUPAC name is 2-hydroxybenzoic acid. It consists of a hydroxyl group (-OH group) attached to the orthro in relation to the carboxylic acid group (-COOH group) present in the benzene ring.
The given molecular weight (or molar mass) of Acetylsalicylic acid is given as 138.12 g / mol.
NCERT Chemistry Notes :
Physical Properties of Acetylsalicylic acid
• Acetylsalicylic acid exists as glossy white or colourless and doorless crystals at room temperature
• The tongue taste of Acetylsalicylic acid is acrid
• The salting and melting point of Acetylsalicylic acid is 211 ° C and 315 ° C, respectively
• The brightest point of Acetylsalicylic acid is 157 ° C
• The concentration of Acetylsalicylic acid is 1.44 to 20 ° C
• Its vapor pressure is 8.2 x 10-5 mm Hg at 25 ° C
• Its LogP is 2.26
• It is the practice of color-correction when exposed to direct sunlight due to its photochemical degradation
• When damaged, it emits irritating fumes and smells that are nutritious
• Its fire temperature is 3.026mj / mol at 25 ° C
• The pH of the complete solution of Acetylsalicylic acid is2.4
• Its pKa (dissociation constant) is 2.97
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Chemical Properties of Acetylsalicylic acid (Acetylsalicylic acid Reaction) / What is aspirin?
Formulation of aspirin structure: In the field of medicine, the most important response associated with use of acetylsalicylic acid is the production of aspirin is also known as acetylsalicylic acid, one of most widely used analgesic and thinning agents. Here, in this given reaction, acetylsalicylic acid is reacted with acetic anhydride in a given acidic environment that even leads to acetylation of the hydroxyl group that is present in acetylsalicylic acid, and it thereby leads to the production of acetylsalicylic acid (aspirin) the mass production of aspirin and must be removed from the resulting product by several refining processes. Aspirin chemical name or aspirin is known as acetylsalicylic acid
Esterification Reaction: Since acetylsalicylic acid is an organic acid, it can be converted by natural alcohol groups to produce a new class of natural chemicals, ester names. When acetylsalicylic acid is reacted with methanol in an acidic environment (possibly sulfuric acid) in the presence of heat, the end-of-life reaction occurs with water loss (OH-ion lost in the active carboxylic acid group present in the acetylsalicylic acid molecule and H+ ion is lost in the destruction of the methanol molecule), leading to the formation of methyl salicylate (ester).
Also read :
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Methods of Preparation Acetylsalicylic acid
It is important to know how to prepare aspirin (acetylsalicylic acid). There are two widely used methods of preparing acetylsalicylic acid. These are the ways discussed below:
From Phenol: When phenol is taken with sodium hydroxide, it forms a sodium phenoxide that is allowed to absorb beverages and weight loss.
The Carboxylation reactions are often followed by carbon dioxide, which also leads to the formation of sodium salicylate (acetylsalicylic acid salt). This form of salt is also reacted with an acid (or hydronium ion or any other proton-expressing form) to obtain acetylsalicylic acid.
From methyl salicylate: Methyl salicylate (wintergreen oil) is also a well-known analgesic in the pharmaceutical industry. It can be used to prepare acetylsalicylic acid. In this reaction, methyl salicylate reacts with sodium hydroxide leading to the formation of a acetylsalicylic acid salt, called disodium salicylate, which, in turn, reacts with sulfuric acid, leading to the formation of acetylsalicylic acid.
Related Topics |
Acetylsalicylic acid uses
• Acetylsalicylic acid has many applications, especially in the pharmaceutical industry. The most common and popular use of acetylsalicylic acid is the preparation of an analgesic, called aspirin, which is an acetylated ingredient of acetylsalicylic acid.
• Acetylsalicylic acid is also used to treat acne and psoriasis. The way acetylsalicylic acid is used in the treatment of these conditions is that it releases the keratin content of the skin by breaking the bonds between molecules between two keratin molecules.
• Acetylsalicylic acid is used to treat warts. The way it treats warts infection is almost identical to its keratolytic action. It removes water from the body of skin cells affected by warts when applied to it and thus leads to its breakdown in the body.
• Acetylsalicylic acid is one of the substances used in anti-dan ramp shampoos. This is because acetylsalicylic acid prevents the accumulation of sebum in the pores of the skin and on the surrounding hair follicles.
• Acetylsalicylic acid also shows little antiseptic effect because it is a known bacteriostatic substance. It does not kill existing germs (so it is not an antibacterial agent) but it prevents the growth of germs when used.
• Acetylsalicylic acid also helps to remove blackheads. This is done with acetylsalicylic acid in the same ways as for acne prevention. It does not allow the skin pores to close and those that are already closed can be opened with the use of acetylsalicylic acid in that area.
• Acetylsalicylic acid is also used to treat infections of the worms and the wet form of infection with the tinea pedis (also known as athlete's foot). Acetylsalicylic acid is also used to treat a very rare skin disease, called Ichthyosis, in which the skin becomes dry, scaly, and thick.
Also check-
Frequently Asked Questions (FAQs)
Acetylsalicylic acid, commonly known as aspirin, is used to relieve pain, reduce inflammation, and lower fever; it is also used in low doses to reduce the risk of heart attacks and strokes.
Yes, Ecosprin is a brand name for acetylsalicylic acid (aspirin), commonly used in low doses for cardiovascular protection and to reduce blood clotting.
Yes, acetylsalicylic acid (aspirin) is considered a blood thinner because it inhibits platelet aggregation, reducing the risk of blood clots.
Another name for acetylsalicylic acid is aspirin.
The chemical formula for acetylsalicylic acid is C₉H₈O₄.
Aspirin is chemically named as Acetylsalicylic acid.
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM

