1. What is acetic acid formula?
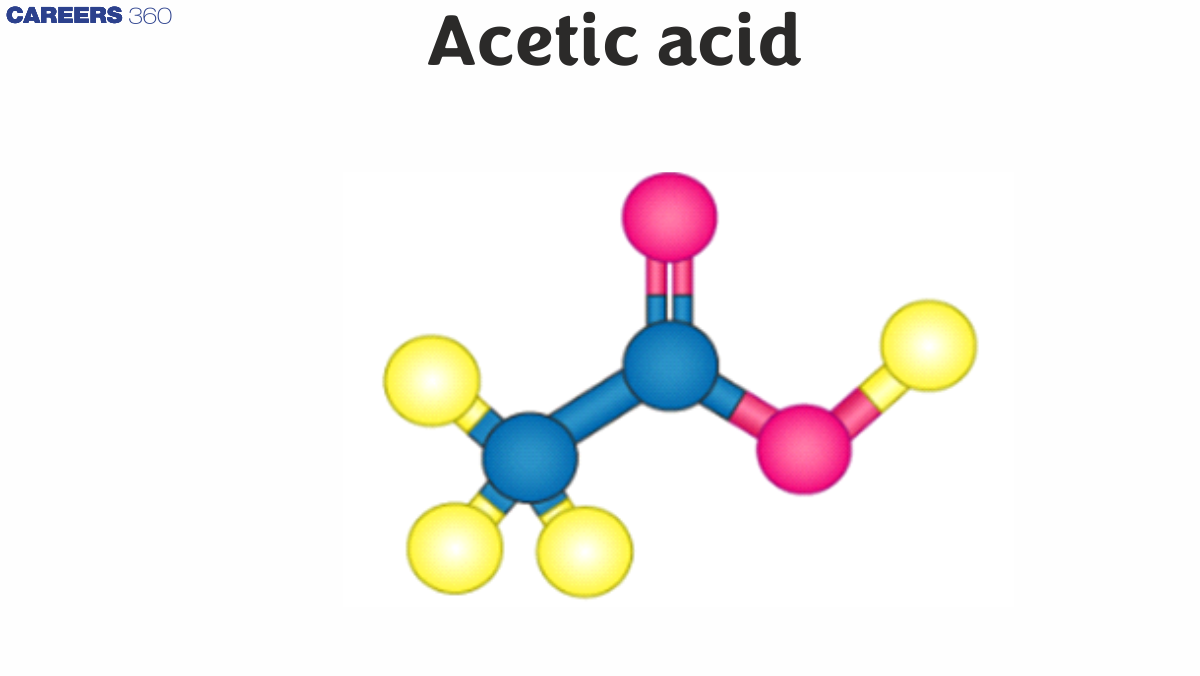
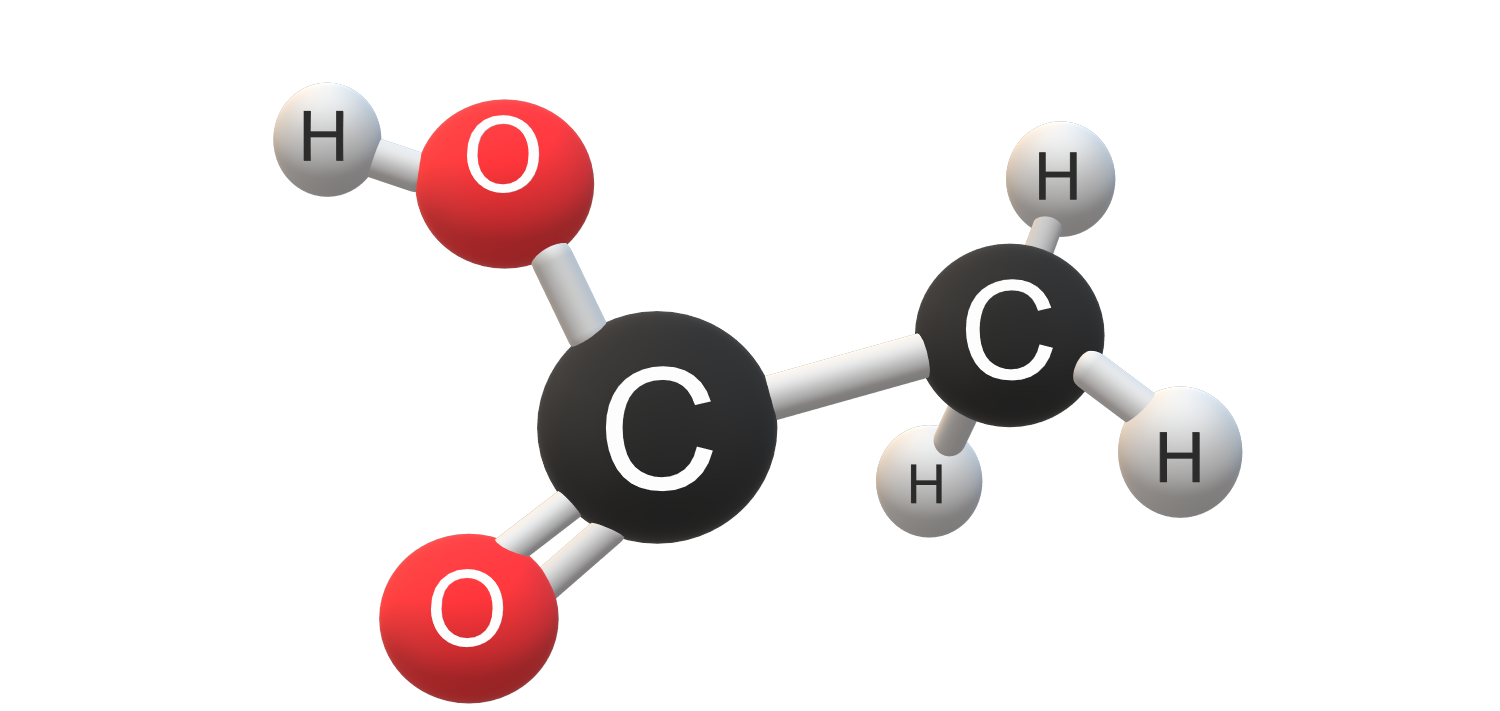
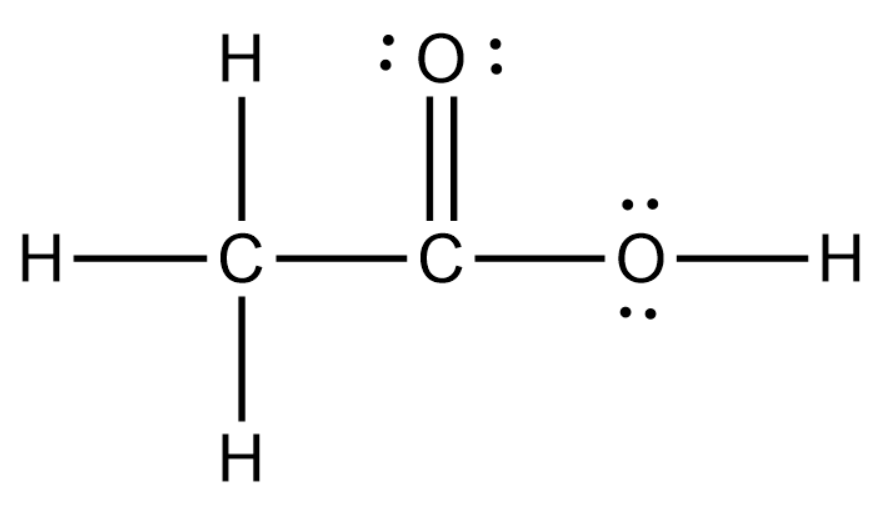
Acetic acid, also known as ethanoic acid has chemical formula CH3COOH.
2. What is the acetic acid used for?
Acetic acid uses are across different fields:
1. Food Industry: Vinegar Production and Food Preservation
2. Chemical Industry: Chemical Reagent
3. Medical Uses: Antiseptic and antibiotics.
4. Household uses: Cleaning Agent
3. What is the common name of CH₃COOH?
The common name of CH₃COOH is acetic acid.
4. Is acetic acid safe?
Acetic acid is generally safe when used in diluted forms, such as in vinegar (about 4-8% concentration). It is commonly used in food, cleaning, and other household applications without causing harm. However, safety depends on its concentration as at higher concentration, it can be an eye, skin, and mucous membrane irritant.
5. How does the structure of acetic acid contribute to its properties?
Acetic acid's structure consists of a carboxyl group (-COOH) attached to a methyl group (-CH3). The carboxyl group is responsible for its acidic properties and ability to form hydrogen bonds. This structure allows acetic acid to participate in various reactions and gives it unique properties like its ability to form dimers in the gas phase.
6. Why is acetic acid considered a weak acid?
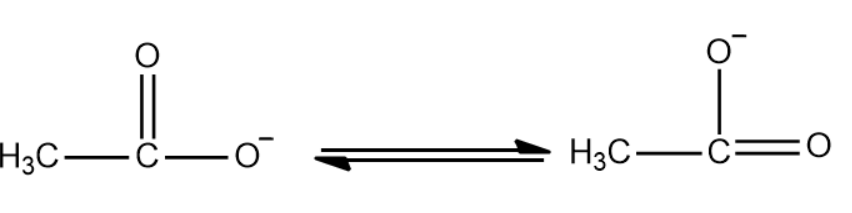
Acetic acid is considered a weak acid because it only partially dissociates in water. This means that in an aqueous solution, most acetic acid molecules remain intact, with only a small fraction ionizing to produce hydrogen ions (H+) and acetate ions (CH3COO-). This partial dissociation results in a relatively low concentration of H+ ions compared to strong acids.
7. What is the pKa of acetic acid, and what does this value indicate?
The pKa of acetic acid is approximately 4.76 at room temperature. This value indicates the acid's strength - the lower the pKa, the stronger the acid. A pKa of 4.76 means that acetic acid is a weak acid, as it doesn't fully dissociate in water. This pKa value is important for understanding acetic acid's behavior in solution and its role in buffer systems.
8. How does acetic acid behave differently in polar and non-polar solvents?
In polar solvents like water, acetic acid can ionize partially and form hydrogen bonds with the solvent molecules, leading to good solubility. In non-polar solvents, acetic acid tends to form dimers through hydrogen bonding between two acetic acid molecules, which can affect its reactivity and physical properties in these environments.
9. What is the significance of acetic acid's ability to form dimers?
Acetic acid's ability to form dimers, especially in the gas phase and non-polar solvents, is significant because it affects its physical properties and reactivity. Dimerization occurs through hydrogen bonding between two acetic acid molecules, creating a cyclic structure. This property influences acetic acid's vapor pressure, boiling point, and behavior in different solvents.
10. What is the difference between acetic acid and ethanoic acid?
Acetic acid and ethanoic acid are the same compound. "Ethanoic acid" is the systematic IUPAC name, while "acetic acid" is the common name. Both refer to CH3COOH. This dual nomenclature is common in organic chemistry and highlights the importance of understanding both systematic and common naming conventions in the field.
11. What is the significance of acetic acid's ability to form a cyclic dimer?
Acetic acid's ability to form a cyclic dimer through hydrogen bonding is significant in understanding its physical properties and behavior, especially in the gas phase and non-polar solvents. This dimerization affects its vapor pressure, boiling point, and reactivity. The cyclic dimer structure provides insight into the nature of hydrogen bonding and molecular association in carboxylic acids.
12. How does acetic acid form hydrogen bonds, and why is this important?
Acetic acid forms hydrogen bonds through its -OH group in the carboxyl portion. The hydrogen atom, being slightly positive, can interact with electronegative atoms like oxygen in other acetic acid molecules or water. This hydrogen bonding is crucial for understanding acetic acid's physical properties, such as its relatively high boiling point and its ability to mix well with water.
13. What is glacial acetic acid, and how does it differ from regular acetic acid?
Glacial acetic acid refers to pure, water-free acetic acid. It's called "glacial" because it forms ice-like crystals below 16.7°C (its melting point). Glacial acetic acid is 99.5% or more pure, whereas regular acetic acid typically refers to aqueous solutions of varying concentrations. Glacial acetic acid is more corrosive and reactive than dilute solutions and is used in various industrial processes.
14. How does temperature affect the dissociation of acetic acid in water?
Temperature affects the dissociation of acetic acid in water by influencing its ionization constant (Ka). Generally, as temperature increases, the Ka of acetic acid slightly increases, meaning it dissociates more readily at higher temperatures. This temperature dependence is important in understanding how acetic acid behaves in various environmental and industrial conditions.
15. How does the strength of acetic acid compare to other carboxylic acids?
Acetic acid is generally weaker than carboxylic acids with electron-withdrawing groups near the carboxyl group, but stronger than those with electron-donating groups. For example, it's weaker than formic acid (HCOOH) but stronger than propionic acid (CH3CH2COOH). The strength is related to the stability of the conjugate base - more stable conjugate bases correspond to stronger acids.
16. How does the presence of acetic acid affect the pH of a solution?
Acetic acid lowers the pH of a solution by releasing hydrogen ions (H+) when it dissociates. However, as a weak acid, it doesn't lower the pH as much as a strong acid of the same concentration would. The extent of pH change depends on the concentration of acetic acid and its degree of dissociation. Understanding this relationship is crucial for predicting and controlling pH in various chemical and biological systems.
17. What is the environmental impact of acetic acid production and use?
The environmental impact of acetic acid varies depending on production methods and usage. Modern production often uses sustainable methods like bacterial fermentation of ethanol. However, some industrial processes can have environmental concerns, including energy consumption and potential for spills. In use, acetic acid is generally considered biodegradable and less harmful than many other industrial chemicals, but proper handling and disposal are still important to minimize environmental impact.
18. What is the role of acetic acid in the production of polyethylene terephthalate (PET)?
Acetic acid plays an indirect but crucial role in the production of polyethylene terephthalate (PET), a common plastic. It's used in the production of terephthalic acid, one of the monomers for PET synthesis. Acetic acid is produced as a byproduct in the oxidation of p-xylene to terepht
19. What is the role of acetic acid in the acetylation of alcohols?
Acetic acid plays a crucial role in the acetylation of alcohols, a process where an acetyl group (CH3CO-) is added to an alcohol. While acetic acid itself is not typically used directly, its derivative, acetic anhydride, is commonly employed. The reaction produces an ester (specifically, an acetate ester) and water. This process is important in organic synthesis, particularly in the production of aspirin from salicylic acid.
20. How does acetic acid participate in Fischer esterification?
In Fischer esterification, acetic acid reacts with an alcohol to form an ester and water. This reaction is typically acid-catalyzed and reversible. The mechanism involves protonation of the carbonyl oxygen, nucleophilic attack by the alcohol, proton transfer, and loss of water. Understanding this reaction is fundamental in organic synthesis and demonstrates the versatility of carboxylic acids in forming various derivatives.
21. What is the role of acetic acid in the formation of acetyl-CoA in biochemistry?
In biochemistry, acetic acid (in its activated form as acetyl-CoA) plays a crucial role in metabolism. Acetyl-CoA is formed when the acetyl group from acetic acid is attached to coenzyme A. This compound is central to the citric acid cycle and fatty acid metabolism. Understanding the chemistry of acetic acid is essential for comprehending these fundamental biochemical processes.
22. How does acetic acid contribute to the taste and aroma of vinegar?
Acetic acid is primarily responsible for the sour taste of vinegar. Its low molecular weight allows it to easily vaporize, contributing to vinegar's pungent aroma. The concentration of acetic acid directly affects the intensity of vinegar's flavor and smell. This relationship between chemical structure and sensory properties is an interesting aspect of food chemistry and flavor science.
23. How does acetic acid behave as a ligand in coordination chemistry?
In coordination chemistry, acetic acid can act as a ligand, binding to metal ions through its carboxylate group. It can coordinate in different ways: monodentate (through one oxygen atom), bidentate (through both oxygen atoms), or as a bridging ligand between two metal centers. This ability to form metal complexes is important in various catalytic processes and in understanding the behavior of acetate salts in solution.
24. How does acetic acid participate in nucleophilic acyl substitution reactions?
Acetic acid can undergo nucleophilic acyl substitution reactions, where the -OH group of the carboxylic acid is replaced by another nucleophile. In these reactions, the carbonyl carbon of acetic acid is attacked by a nucleophile, forming a tetrahedral intermediate. The -OH group then leaves, and a new group is attached. This type of reaction is fundamental in many organic syntheses, including esterification and amide formation.
25. How does acetic acid contribute to the preservation of food?
Acetic acid contributes to food preservation by lowering the pH of the food, creating an acidic environment that inhibits the growth of many bacteria, yeasts, and molds. Additionally, the acetate ion can penetrate microbial cell membranes, further disrupting their growth. This preservative action is why vinegar (dilute acetic acid) is widely used in pickling and food preservation.
26. What is the role of acetic acid in buffer solutions?
Acetic acid, along with its conjugate base (acetate ion), forms an important buffer system. A buffer solution resists changes in pH when small amounts of acid or base are added. The acetic acid/acetate buffer is effective near its pKa (4.76), making it useful in biological systems and laboratory applications where maintaining a stable pH around this value is crucial.
27. What is acetic acid and why is it important in organic chemistry?
Acetic acid (CH3COOH) is a simple carboxylic acid and the main component of vinegar. It's important in organic chemistry because it serves as a model compound for understanding carboxylic acid properties and reactions. Acetic acid is widely used in industry for producing polymers, solvents, and other chemicals.
28. What is vinegar, and how is it related to acetic acid?
Vinegar is a dilute aqueous solution of acetic acid, typically containing 5-8% acetic acid by volume. It's produced by the fermentation of ethanol by acetic acid bacteria. The acetic acid in vinegar is responsible for its sour taste and preservative properties. Understanding the chemistry of acetic acid is crucial for comprehending vinegar's properties and applications.
29. How does acetic acid participate in esterification reactions?
Acetic acid participates in esterification reactions by combining with an alcohol in the presence of an acid catalyst. The -OH group of the carboxylic acid is replaced by an -OR group from the alcohol, forming an ester and water. This reaction is reversible and is a key process in organic synthesis, used to produce various esters with applications in fragrances, flavors, and plastics.
30. What is the mechanism of acetic acid's reaction with sodium bicarbonate?
When acetic acid reacts with sodium bicarbonate (baking soda), it undergoes an acid-base neutralization reaction. The hydrogen ion from acetic acid combines with the bicarbonate ion to form carbonic acid, which quickly decomposes into carbon dioxide and water. This reaction produces the characteristic fizzing observed when vinegar is mixed with baking soda. The overall reaction is: CH3COOH + NaHCO3 → CH3COONa + H2O + CO2.
31. What is the importance of acetic acid in the production of vinyl acetate?
Acetic acid is a key reactant in the production of vinyl acetate, an important industrial chemical. Vinyl acetate is produced by reacting acetic acid with ethylene and oxygen in the presence of a palladium catalyst. This reaction is crucial because vinyl acetate is the precursor to polyvinyl acetate (PVA), which is used in adhesives, paints, and other polymer products.
32. What is the significance of acetic acid's boiling point compared to other organic compounds?
Acetic acid has a relatively high boiling point (118°C) compared to other organic compounds of similar molecular weight. This high boiling point is due to strong hydrogen bonding between acetic acid molecules. The ability to form these intermolecular hydrogen bonds distinguishes carboxylic acids like acetic acid from other organic compounds and affects their physical properties, including solubility and vapor pressure.
33. How does acetic acid behave in a titration with a strong base?
In a titration with a strong base like sodium hydroxide, acetic acid acts as a weak acid. The titration curve shows a gradual increase in pH initially, followed by a steep rise near the equivalence point. The equivalence point occurs at a pH above 7 due to the basic nature of the acetate ion produced. This titration behavior is important for understanding acid-base reactions and for analytical chemistry applications.
34. How does acetic acid interact with metals, and why is this important?
Acetic acid can react with many metals to form metal acetates, often releasing hydrogen gas in the process. This reaction is more pronounced with more reactive metals like zinc or magnesium. The interaction of acetic acid with metals is important in understanding corrosion processes, especially in food processing equipment or when storing vinegar. It also has applications in the synthesis of various metal acetate compounds used in industry and research.
35. What is the role of acetic acid in the production of cellulose acetate?
Acetic acid is crucial in the production of cellulose acetate, a important polymer used in textiles, films, and other applications. In this process, cellulose reacts with acetic anhydride (derived from acetic acid) in the presence of sulfuric acid as a catalyst. The hydroxyl groups on cellulose are replaced by acetyl groups, altering its properties. This reaction demonstrates the importance of acetic acid derivatives in polymer chemistry.
36. How does the concentration of acetic acid affect its properties and reactivity?
The concentration of acetic acid significantly affects its properties and reactivity. More concentrated solutions exhibit stronger acidic properties, lower pH, and increased reactivity in acid-catalyzed reactions. Dilute solutions behave more like water in terms of physical properties. The relationship between concentration and properties is non-linear due to the weak acid nature of acetic acid, which is important in understanding its behavior in various applications.
37. What is the significance of acetic acid's dissociation constant (Ka)?
The dissociation constant (Ka) of acetic acid, approximately 1.8 × 10^-5 at room temperature, quantifies its strength as an acid. This value indicates that acetic acid is a weak acid, as only a small fraction of molecules dissociate in solution. The Ka is crucial for calculating pH, understanding buffer systems, and predicting the behavior of acetic acid in various chemical reactions and equilibria.
38. How does acetic acid behave in non-aqueous solvents?
In non-aqueous solvents, acetic acid's behavior can differ significantly from its behavior in water. In aprotic solvents, it tends to form dimers through hydrogen bonding. In some organic solvents, it may act as a stronger acid due to the lack of stabilizing water molecules. This behavior in different solvents is important in understanding reaction mechanisms and solvent effects in organic chemistry.
39. What is the role of acetic acid in the synthesis of acetylsalicylic acid (aspirin)?
Acetic acid, in the form of acetic anhydride, is crucial in the synthesis of acetylsalicylic acid (aspirin). It reacts with salicylic acid in an esterification reaction, where the acetyl group from acetic anhydride replaces the hydrogen of the phenolic hydroxyl group in salicylic acid. This reaction demonstrates the importance of acetic acid derivatives in pharmaceutical synthesis and illustrates the principle of prodrug design.
40. How does acetic acid contribute to the formation of esters in wine and other fermented beverages?
In wine and other fermented beverages, acetic acid contributes to ester formation through a process called esterification. Acetic acid reacts with alcohols present in the beverage (primarily ethanol) to form various esters, which contribute to the aroma and flavor profile. This process occurs slowly over time and is one reason why some wines improve with age. Understanding this chemistry is crucial in oenology and food science.
41. How does acetic acid interact with amino acids and proteins?
Acetic acid can interact with amino acids and proteins in several ways. It can protonate the amino groups, affecting the overall charge and solubility of proteins. In more concentrated forms, it can denature proteins by disrupting hydrogen bonds. Acetic acid can also form esters with the hydroxyl groups of certain amino acids. These interactions are important in food chemistry, biochemistry, and protein purification techniques.