Unit of Wavelength - Definition, SI Unit, FAQs
Wavelength is the length of one cycle of a wave, which is the distance. The SI unit of wavelength is meter (m), and the wavelength symbol is λ. In this article we will learn in detail about waves, types of waves, wavelength units, what the unit of wavelength is, and how you measure wavelength.
What is a wave?
A wave is the propagation of disturbance from one point to another in a regular and organized manner. In a scientific manner, a wave is a transfer of energy in a medium. When energy moves through any matter like solid, liquid, or gas, then the particles of these matters get disturbed, which results in the formation of waves.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is a wave?
- Types of waves
- What is wavelength, and what is the unit of wavelength?
- Units of wavelength
- Wavelengths of different waves in meters
- How to measure wavelength?

Some energies need a medium to travel, while some energies do not need a medium to travel from one point to another. Waves are divided into two types: longitudinal waves and transverse waves.
Longitudinal waves: The waves that oscillate in parallel with respect to the propagation of waves. They can travel through solids, liquids, and gases as well.
Transverse waves: The waves that oscillate perpendicular with respect to the propagation of waves. These travel through the surface of liquids and gases, but they can not travel through gases.
Types of waves
There are 3 types of waves:
- Mechanical waves
- Electromagnetic waves
- Matter waves
Each of these is defined briefly in the section given below:
Mechanical waves
Mechanical waves are defined as the waves that require a medium to travel. They transfer energy through the medium by oscillating the particles. For example,
- Sound waves
- Seismic waves
- Water waves
Electromagnetic waves
Electromagnetic waves are named so because they are constituted of electric and magnetic fields. These waves propagate in the space as oscillating electric and magnetic fields. As these waves do not need any medium to travel, they can travel through a vacuum. The electromagnetic waves travels with the speed of light, i.e.,
For example,
- Radio waves
- Infrared waves
- UV radiation
- Gamma rays
- Visible waves
- Microwaves
Matter waves
The matter waves, also known as de Broglie waves, are the waves that follow the quantum mechanical statement that all matter shows behavior like waves. This leads to the concept of the dual behavior of particles.
For example, particles like electrons and photons show dual nature.
What is wavelength, and what is the unit of wavelength?
Let us define wavelength in physics: a wave is a theoretical representation of energy propagating in a medium or vacuum. This kind of energy is also called a disturbance that propagates in a certain medium, whether it is a solid, liquid, or gas. For example, sound energy. Only a small amount of energy does not require the medium to move, so it can move in a vacuum. For example, light energy.
We know transverse waves travel in consecutive successive crests and troughs, while longitudinal waves travel in successive compressions and rarefactions.
In the case of a transverse wave, the length of successive crests and troughs is called its wavelength, while in the case of a longitudinal wave, the length of successive compressions and rarefactions is called its wavelength.
Wavelength is the length of a wave from the maximum point of a peak to the maximum point of the adjacent peak or the lowermost point of a valley to the lowermost point of the adjacent valley.
It is denoted by a symbol called lambda (λ), and the unit of lambda is the meter. According to the definition given, we know that wavelength is length, so the SI unit of wavelength should be equal to the SI unit of length. We know that the SI unit of length is meters, so the SI unit of wavelength is also meters.
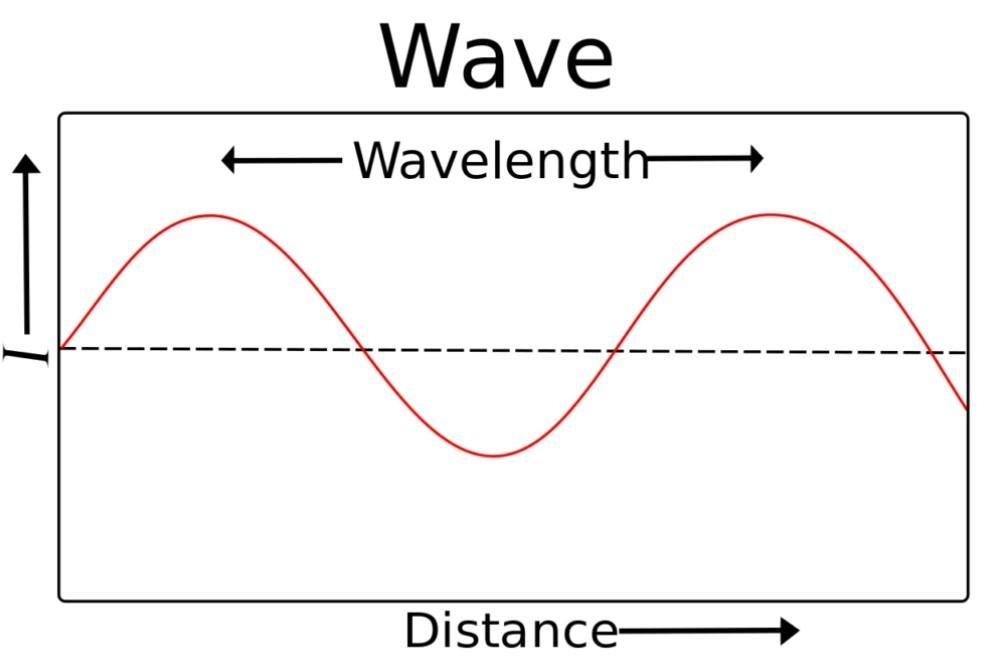
Light is a form of electromagnetic radiation, a field associated with light energy. Light is a very general term because it can be anything from a simple light bulb to a microwave oven. Some characteristics of light include wavelength and frequency. Frequency (usually measured in Hertz) is the number of waves in a given time. The wavelength (usually measured in nanometers) is the distance between two points in a wave. Frequency and wavelength have a positive and negative relationship. For example, if two waves are moving at the same speed, they are negatively correlated. Waves of shorter wavelengths have higher frequencies, and longer wavelengths have lower frequencies. Frequency and wavelength may be related to the speed of light. Light moves at a speed of 3.00 x
Just as wavelength and frequency are associated with light, they are also associated with energy. The tinier the wavelength, the greater the frequency and the more the energy. The lengthier the wavelength, the lesser the frequency and the lesser the energy. The energy equation is E = h
Also read
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
Units of wavelength
As mentioned earlier, the SI unit of wavelength is meters. Some wavelengths are very short, and some wavelengths are very long. Solving various numerical problems also requires larger and smaller unit lengths. Therefore, we use an exponent of 10 to measure large properties, while a negative exponent is used to measure shorter wavelengths.
The following are all units of wavelength:
- Kilometer, abbreviated as km, is equivalent to
- Megameter, abbreviated as Mm, is equivalent to 1000000 m or
- Gigameter, abbreviated as Gm, is equivalent to 1000000000 m or
- Terameter, abbreviated as Tm, is equivalent to
- Petametre, abbreviated as Pm, is equivalent to
- Decimetre, abbreviated as dm, is equivalent to
- Centimetre, abbreviated as cm, is equivalent to
- Millimetre, abbreviated as mm, is equivalent to
- Micrometer, abbreviated as
- Nanometre, abbreviated as nm, is equivalent to
Related Topics Link, |
Wavelengths of different waves in meters
Light is a type of energy that travels in waves; it is made when matter is heated up or gains energy. Excess energy is released in part as light; this energy is called electromagnetic radiation. When we talk about light, we usually mean visible light, which is the light we can see with our eyes, but there are more types of electromagnetic radiation that are invisible to us, including radio waves, microwaves, x-rays, and gamma rays. Scientists can detect and measure invisible radiation by using special tools. Collectively with visible light, all these kinds of radiation are called the electromagnetic spectrum. All electromagnetic radiation moves in waves, but unlike types have unlike wavelengths. The wavelength of electromagnetic emission or light is associated with how much energy it has.
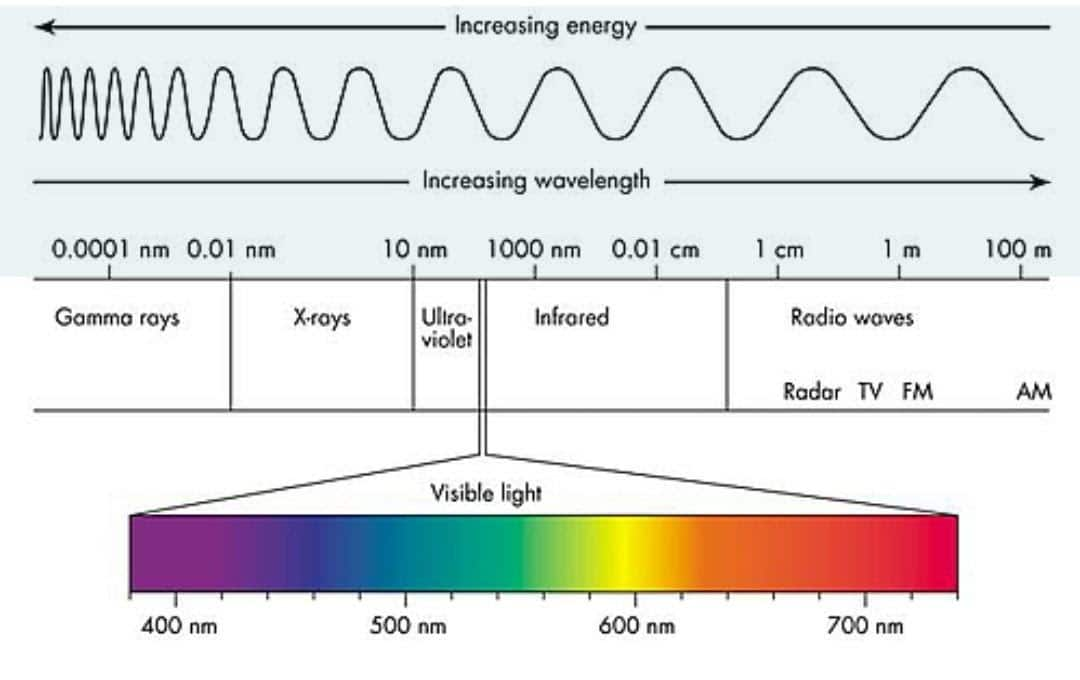
Now let us take a look at the wavelength of these electromagnetic radiations here. Wavelength is measured in m and nm.
Gamma rays: Gamma rays have the shortest wavelength. It is less than 0.001 nanometers or 1012 meters.
X-ray: The wavelength range of X-rays is 0.001-10 nm.
Ultraviolet: The wavelength range of ultraviolet is 10–400 nm.
Visible light: The wavelength of visible light is within the range of 400–700 nm.
Infrared: The infrared wavelength range is 700 nm to 1 mm.
Radio waves: Radio waves have the lengthiest wavelength. Its length exceeds 1 mm or 0.001 meters.
Also Read:
- NCERT Solutions for Class 11 Physics Chapter 15 Waves
- NCERT Exemplar Class 11 Physics Solutions Chapter 15 Waves
- NCERT notes Class 11 Physics Chapter 15 Waves
How to measure wavelength?
To understand wavelength measurement and to know clearly about how to measure wavelength, let us take an example.
Calculate the wavelength of radiation with a frequency of 5 X
We know,
The frequency of the radiation is
The speed of light in a vacuum is
⇒
⇒
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes:
Frequently Asked Questions (FAQs)
The wavelength will decrease.
λ=c/v =speed of light/frequency
= 3 X
Wavelength and frequency of a wave are inversely proportional. As wavelength increases, the frequency decreases.
Meters and Hertz are the SI unit of wavelength and frequency respectively.
The wavelength range of X-ray is 0.001-10 nm.
The wavelength of matter wave depends upon the mass, velocity, and momentum of the particle.
The red color has the longest wavelength of about 700 nm in the visible range.
The lower wavelength waves have the higher frequency and the higher energy as well, while the waves with higher wavelength hav the lower frequency and lower energy by the relation
Also Read
02 Jul'25 06:19 PM
02 Jul'25 05:07 PM
02 Jul'25 05:04 PM
02 Jul'25 05:00 PM
02 Jul'25 04:59 PM
02 Jul'25 04:53 PM
02 Jul'25 04:48 PM
02 Jul'25 04:47 PM
02 Jul'25 04:45 PM

