Frequency and Wavelength - Definition, Wavelength of Light, Examples, FAQ
When we talk about waves, it may be a transverse wave like light or a longitudinal wave like sound, we come across some terms which are inevitably connected with the wave motion. These are- frequency and wavelength of a wave, time period, amplitude, phase, angular frequency, wave number, angular wave number and so on. However in this article we will mainly discuss frequency and wavelength of a wave in detail along with the other concepts. We will also discuss about ’what is frequency of a wave?’, ‘what is wavelength of a wave?’, ‘wavelength and frequency relationship’, ‘what is wavelength and frequency of light?’, ‘ numericals based on frequency-wavelength relation’, ‘factors affecting frequency and wavelength of a light wave’, ‘frequency of light’ and ‘ energy and wavelength relationship’. But first let us understand ‘what is a wave?’ and ‘what are the types of waves?’.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs

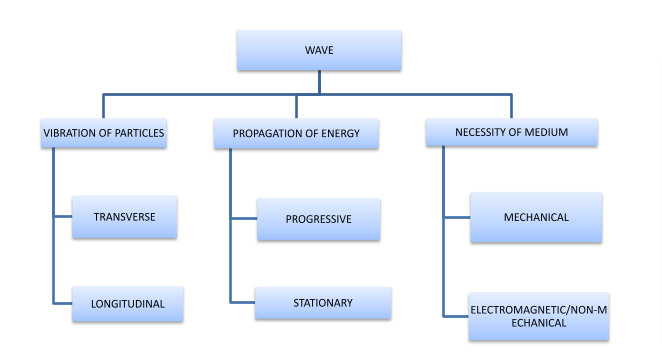
Wave and its classifications
Before understanding the concept of frequency and wavelength of a wave in detail, let us first define the wave.
A wave can be considered as a disturbance that propagates in space which transports energy and momentum from one point of space to another without actual transport of matter.
For example, we consider sound wave motion. When we talk to someone, we actually create disturbance in the air close to our lips. Energy is then transferred to the air particles of this region by either pushing or pulling them. These disturbed particles exert force on the neighboring particles and so on and thus transfer this disturbance from our lips to the other person’s ear without actually transferring any particle from our lips to the other person’s ear.
Classification of waves can be done on the basis of –
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
What is wavelength of a wave?
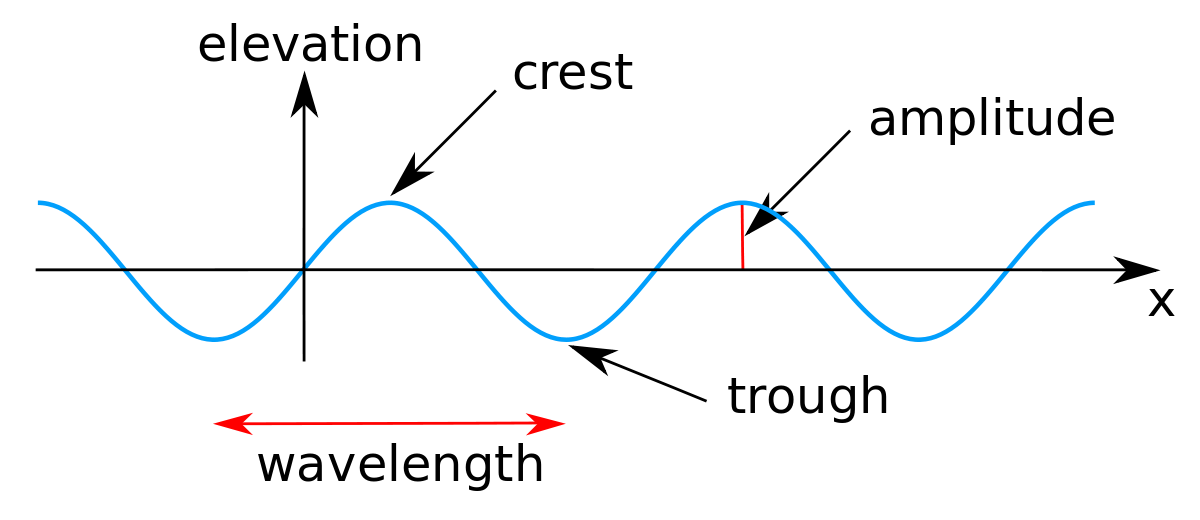
Wavelength of a wave (denoted by lambda λ) is the distance travelled by the wave when one particle of the medium completes one vibration about its mean position or can be defined as the distance between nearest particles of the medium, vibrating in the same phase.
Consider a transverse wave (see figure-1)
 (Fig-1)
(Fig-1)
A crest is a point of the medium, which is raised above the normal position of rest of the particles of the medium while a trough is opposite to crest which is a minimum point of the wave. The wavelength of the wave in this case is the distance between two consecutive crests or troughs.
However, in case of longitudinal waves, particles produce regions of compression (high pressure) and rarefaction (low pressure) and the wavelength in this case is the distance between two consecutive compressions or two consecutive rarefactions.
Unit of wavelength is the same as distance i.e. meter in S.I. unit.
Wave number- Denoted by ѵ,It is defined as the number of waves per unit length of a wave pattern. It is clearly reciprocal of the wavelength of the given wave.
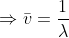 (Equation-1)
(Equation-1)
Angular wave number- Denoted by k, it is product of 2π with 1/λ . Thus,
=>k= 2π× 1/λ (Equation-2)
|
Related Topics Link, |
What is frequency of a wave?
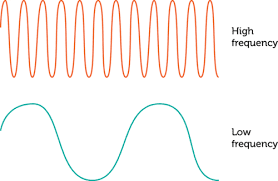
Frequency is defined as the number of periodic motions executed by a body or a particle per second. It is denoted by ν (or n or f). Hence, number of vibrations or number of complete wavelengths by a particle in 1 second is frequency of a wave (see figure-2)
 (Fig-2)
(Fig-2)
Unit of frequency is hertz denoted by Hz. Hence, 1Hz=1 oscillation per second= 1 cps= 1s-1.
Remember that frequency may not be necessarily an integer.
Now, Time period (T) is the time taken by the wave to travel a distance equal to one wavelength. Hence, it is the least interval of time after which the periodic motion of a body repeats itself (see figure-3).
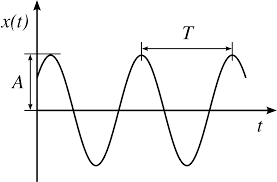 (Fig-3)
(Fig-3)
Unit of time period is second. Thus, clearly the frequency of a wave is reciprocal of time period.
ν=1/T (Equation-3)
Angular frequency of a body which executes periodic motion is nothing but equals to the product of frequency of the body with factor 2π. It is denoted by ω.
Thus, ω=2π×ν=2π/T
=>ω =2π/T (Equation-4)
S.I. unit of angular frequency is rad/s.
NCERT Physics Notes:
Relationship between wavelength and frequency
Since speed equals distance by time, therefore v=λ/T, where λ is the wavelength and T is the time period.
Wavelength and frequency relation can be written as-
V=λν (Equation-5)
=>speed= lambda×frequency
Wavelength and frequency of light
If speed of light is denoted by c, then by equation-5 we can write-
C= λ/T (Equation-6)
Now, we already know that speed of light is measured to be almost equal to 3×108m/s. Then if we are given either the time period or frequency of the light we are considering, we can calculate the wavelength of light and similarly if we know the wavelength of light we are using, we can calculate frequency. Thus equation-6 also describes the wavelength and frequency relation in case of electromagnetic waves.
The table below shows electromagnetic spectrum arranged by their respective frequency and wavelength. Their photon energies are also specified.
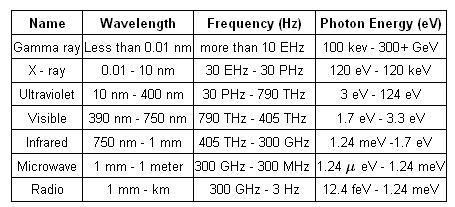
Also read :
- NCERT notes Class 11 Physics Chapter 15 Waves
- NCERT solutions for Class 11 Physics Chapter 15 Waves
- NCERT exemplar Solutions for Class 11th Physics Chapter 15 Waves
Factors affecting frequency and wavelength of a light wave
Both wavelength and velocity of a light wave can change but the frequency of the light wave always stays the same. When an electromagnetic wave enters a denser medium such as glass, its velocity decreases by a factor of the refractive index of that medium (n). Since frequency of an electromagnetic wave never changes, its wavelength is bound to change to account for the change in its velocity. Thus, lower the velocity of the electromagnetic wave is, the shorter its wavelength will be. This can be clearly seen by equation-5 where, if we keep the frequency constant, the velocity of the electromagnetic wave is directly proportional to its wavelength. The above case is nothing but the phenomenon of refraction of light (see figure-4). This is one of the many ways by which we can change the wavelength or velocity of an electromagnetic wave.
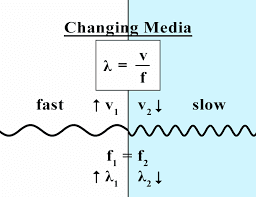 (Fig-4)
(Fig-4)
Wavelength and energy relationship
Wien's radiation law and Raleigh-Jean law tried to establish wavelength and energy relationship but the former one came up with an empirical formula that was only true for lower wavelengths and the later one came up with an equation which was only true for larger wavelengths or lower frequencies. The vice versa for both of these equations gave random and wrong values. Thus, Planck solved this problem by modifying classical mechanics and laying the basis for Quantum Mechanics. He found a whole new equation which states that energy emitted or absorbed from radiation at frequency ν is equal to nhν, n=1, 2,...
That implies, emission or absorption of energy can be in multiples of hν.
Thus, wavelength and energy relationship can be written as-
E=nhv, where h=Planck’s constant. If n=1, E=hν (Equation-6)
E=nhc/λ (Equation-7) 5×1014s-1
The above equation is strictly for photons in electromagnetic waves. However, we know that light has dual nature i.e. both wave and particle nature. Thus, for matter waves, de broglie found the equation as-
λ=h/P, where P is the momentum of the particle present in the wave and lambda is its wavelength. (Equation-7)
Also check-
Frequently Asked Questions (FAQs)
c=λf=>f=c/λ=3×108 / (200×10-9)Hz=1.5×1014Hz
No, reflection of light doesn’t change its wavelength.
By equation-7, λ=hc/E = 12.4kevÅ/1kev ( as hc=12.4kevÅ)
=>λ=12.4Å
Now,f=c/ λ=3×108/12.4×10-10m =2.42×1014Hz
From equation-6 and equation-4, E=hω/2π
Also Read
02 Jul'25 06:19 PM
02 Jul'25 05:07 PM
02 Jul'25 05:04 PM
02 Jul'25 05:00 PM
02 Jul'25 04:59 PM
02 Jul'25 04:53 PM
02 Jul'25 04:48 PM
02 Jul'25 04:47 PM
02 Jul'25 04:45 PM

